Farnesol, a natural sesquiterpene alcohol in essential oils, was found to have potential for alleviating massive inflammation, oxidative stress and lung injury. However, effects of farnesol supplementation on allergic asthma remain unclear.
ObjectivesTo clarify the puzzle, this work investigates the effects of farnesol on allergic asthma using an ovalbumin (OVA)-sensitised and challenged mouse model.
MethodsFarnesol was administered to OVA-sensitised and challenged mice for 5 weeks. Three farnesol doses, namely 5, 25 and 100mg farnesol/kg BW/day, non-sensitised control, dietary control, and positive control (dexamethasone 3mg/kg BW by gavage) were included. Sera and bronchoalveolar lavage fluids from the experimental mice were collected to measure farnesol concentrations, serum lipid profiles, antibody titres, differential cell counts or Th1/Th2 cytokines levels.
ResultsThe results showed that farnesol supplementation increased serum farnesol concentration dose-dependently, significantly increased (P<0.05) OVA-specific IgG2a/IgE antibody titre ratios, but decreased total IgE levels. Farnesol supplementation markedly reversed the aberrated LDL-c/HDL-c and HDL-c/TC ratios in the sera of asthmatic mice, suggesting that farnesol supplementation might ameliorate serum lipid profiles in the OVA-sensitised and challenged mice.
ConclusionOur results evidenced that farnesol supplementation might improve serum allergic antibody titres and lipid profiles in asthmatic mice.
Allergic asthma is a chronic airway inflammatory disease with increased infiltration of eosinophils into the airway, over-expression of T helper type 2 (Th2) cytokines such as IL-4, IL-5 and IL-13, elevated serum immunoglobulin (Ig)E levels, mucus hyper-secretion by goblet cells, resulting in airway hyper-responsiveness and inflammation.1,2 Patients of allergic asthma have an imbalance between Th1 and Th2 immune responses; nowadays it is recognised that allergic asthma is a Th2-skewed disease.3 Several studies have indicated that Th2-skewed diseases can be effectively improved by enhancing Th1-favoured immune responses,4 or by mediating Th2 subpopulation and eosinophils differentiation, as well as modulating B cell proliferation and IgE isotype switching.5,6 In Th1-favoured immune responses Th1 cells play the leading role that secrete interferon (IFN)-γ, tumour necrosis factor (TNF)-α/β and interleukin (IL)-2 to inhibit over-expression of Th2 cytokines. Regulation of an imbalance between Th1 and Th2 immune responses in allergic asthmatic patients using bioactive compounds is a good strategy for ameliorating an allergic injury.
Although numerous drugs such as steroids, leukotriene inhibitors, mast cell stabilisers and β2-adrenergic agonists have been utilised to treat asthma currently,7 about 50% patients remain difficult to improve and even suffer from adverse side effects, further suggesting that these drugs are not suitable for patients with severe asthma.8 It is most important to prevent the early manifestations of the disease and thus to suppress its evolution into severe asthma.9 It is therefore necessary to seek other new therapeutic methods or alternative agents for improving allergic asthma. The morbidity of allergic asthma is increasing and becoming a problem of public health in the world. Natural plant compounds are suggested for use in preventing or treating asthma.5 Traditional herbal medicines, health foods and their possible active compounds that can be ingested daily shed a light for preventing or treating allergic asthma.
Farnesol is a sesquiterpene alcohol that exists widely in fruits such as peaches, vegetables such as tomatoes and corn, herbs such as lemon grass and chamomile, as well as the essential oils of ambrette seeds, and citronella.10,11 It can also be produced endogenously in animal cells from farnesyl pyrophosphate, the precursor of squalene in the cholesterol biosynthetic pathway.10 Farnesol has been widely used in cosmetics, pharmaceuticals, industrial materials and as a material for carotenoid, tocopherol or co-enzyme Q10 syntheses.12,13 Recently, farnesol was found to have potential for alleviating massive inflammation, oxidative stress and lung injury induced by intratracheal instillation of cigarette smoke extract in rats.14 Farnesol also ameliorated 1,2-dimethylhydrazine induced oxidative stress, inflammation and apoptosis in the colon of Wistar rats.11 Most recently, farnesol was found to exhibit a relative Th1-inclination and anti-inflammatory property to immune cells in vitro, suggesting that it may be applied to improve Th2-skewed allergic asthma.15
Ovalbumin (OVA) is a known allergen to induce allergic asthma. OVA sensitisation and challenge to experimental mice could induce both systemically and locally allergic inflammation responses including changes in serum OVA-specific antibody titres and cells infiltration into the airways.16 To investigate the possible effects of farnesol supplementation on allergic asthma, an OVA-sensitised and challenged asthmatic mouse model was established in the present study. Farnesol at different doses was administered to the OVA-sensitised and challenged mice for 5 weeks. Changes in serum farnesol concentrations and antibody tilters, as well as differential cell counts and Th1/Th2 cytokines levels in bronchoalveolar lavage fluid (BALF) of the experimental mice were measured.
Materials and methodsSampleFarnesol (C15H26O) is a natural sesqui-terpenoid in essential oils found in many plants.11 In this study, farnesol (Sigma, St. Louis, MO, USA) was purchased at the highest available purity (>95%, a mixture of isomers).
Experimental animals and dietary groupsThe experimental feed was prepared according to the recommendation of the American Institute of Nutrition AIN-76 that satisfies the nutritional requirement for mouse growth and varied only in farnesol composition.17 Three farnesol doses, including low dose (0.003%), medium dose (0.017%) and high dose (0.067%), were added into the AIN76 feed.18 The components of each feed were prepared by thoroughly mixing and storing at −20°C. Approximately, 3g of AIN76 feed were consumed by each individual mouse with 20g body weight (BW) per day. The designed farnesol low (FL), medium (FM), and high (FH) doses corresponded to 5, 25 and 100mg farnesol/kg BW/day, respectively. It could be estimated that farnesol supplementation at the indicated doses might not produce significant energy in vivo. The energy contribution of each experimental diet was 67.5% from carbohydrate, 20.8% from protein and 11.7% from fat. The calorie density of each diet was 3.85kcal/g. The animal use protocol listed below was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC), National Chung Hsing University, Taiwan, ROC. Generally, both male and female animals were administrated into different animal models. Male and female animals have similar immune responses in many situations; however only female mice were used in this study.19 Unfortunately, in the present study the experimental mice were not controlled for testing during a particular phase of the oestrous cycle. The female BALB/cByJNarl mice (7 weeks old) were obtained from the National Laboratory Animal Center, National Applied Research Laboratories, National Science Council in Taipei, ROC and maintained in the Department of Food Science and Biotechnology at National Chung Hsing University College of Agriculture and Natural Resources in Taichung, Taiwan, ROC. The animal room was kept on a 12-h light and 12-h dark cycle. Constant temperature (25±2°C) and ambient humidity (50–75%) were maintained. The mice were housed and kept on a chow diet (laboratory standard diet, Diet MF 18, Oriental Yeast Co., Ltd., Osaka, Japan) to acclimate for 1 week before feeding the experimental diet. After this equilibrium period, the mice were randomly divided into six groups (n=15) varied by farnesol doses and sensitised treatments (Day 0): non-sensitised control (treated with phosphate-buffered saline (PBS) and alum (Al(OH)3), namely PBS/AL, coded as NSC), dietary control (treated with OVA and alum, namely OVA/AL, coded as DC), farnesol low dose (treated with OVA/AL, supplemented with low dose farnesol about 5mg/kg BW/day, coded as FL), farnesol medium dose (treated with OVA/AL, supplemented with medium dose farnesol about 25mg/kg BW/day, coded as FM), farnesol high dose (treated with OVA/AL, supplemented with high dose farnesol about 100mg/kg BW/day, coded as FH), and positive control (treated with OVA/AL, treated with dexamethasone, coded as PC). It was a long-term study; therefore some of the mice died due to the OVA/AL manipulation, but not the farnesol toxicity, during the late experimental period, and thus the data collected from these dead mice were excluded. The initial average body weight of each group showed no significant differences among groups. Each group was fed with the specified experimental diet for 35 consecutive days ad libitum. Mouse food intake and body weight were measured twice a week during the study period. There were no significant differences in food intake and body weight among groups. To measure changes in antibody titres, experimental mice were bled using a retro-orbital venous plexus puncture to collect approximately 100μL serum samples at Days −1, 7, 21 and 35. All experimental mice were sacrificed at Day 36 after grouping, and then bronchoalveolar lavage fluid (BALF) and the serum were collected for assay.
Sensitisation and airway challengeThe mice (8 weeks old) were sensitised and challenged to induce allergic airway inflammation. Mice were sensitised using an intraperitoneal injection (i.p.) of 0.2mL alum-precipitated antigen containing 8μg of ovalbumin (OVA, albumin chicken egg grade III, Sigma–Aldrich Co., St. Louis, MO, USA) and 2mg Al(OH)3 (Sigma–Aldrich Co., St. Louis, MO, USA) to induce primary immunity and started the specified experimental diets (at Day 0). Two booster injections of this alum–OVA mixture were given 14 and 28 days later, respectively. Non-sensitised control mice received alum-phosphate-buffered saline (PBS, 137mM NaCl (Wako Pure Chemical Industries, Ltd., Osaka, Japan), 2.7mM KCl (Sigma–Aldrich Co., St. Louis, MO, USA), 8.1mM Na2HPO4 (Sigma–Aldrich Co., Steinhein, Germany), 1.5mM KH2PO4 (Sigma–Aldrich Co., St. Louis, MO, USA), pH 7.4, 0.2μm filtered) only. At Days 31 and 34, the OVA-sensitised mice were challenged using aerosolised OVA at a concentration of 5mg OVA per millilitre PBS for 60min (namely, 9:00am for 30min and 4:00pm for 30min). The aerosolised OVA were produced using an ultrasonic nebuliser (sw918, Shinmed, Taipei, Taiwan). Non-sensitised control mice received only PBS. Two hours before aerosolised OVA was administered, the PC group was treated with dexamethasone (DEX, 3mg/kg BW, 0.3mL/mouse, Sigma, St. Louis, MO, USA) by gavage to reduce allergic asthmatic inflammation.20 Two days later (at Day 36), all of the animals were anaesthetised, exsanguinated using retro-orbital venous plexus puncture and immediately euthanised by CO2 inhalation. The sera and bronchoalveolar lavage fluid (BALF) were collected and assayed for cytokines and other inflammatory mediators. Sensitisation and challenge protocols for inducing allergic airway inflammation in mice were performed. There were no significant differences in visceral organ weights between the farnesol supplemented groups and dietary control group, suggesting that farnesol supplementation at the indicated doses shows no toxicity to the experimental mice.
Bronchoalveolar lavage fluid (BALF) collection and cellular differential countsThe experimental mice were anaesthetised with diethyl ether, bled using a retro-orbital venous plexus puncture to collect blood and immediately euthanised by CO2 inhalation. After the mice were euthanised, the airways and the lungs were immediately lavaged aseptically using a cannula through the trachea with five aliquots of 0.6mL Hank's balanced salts solution (HBSS), free of ionised calcium and magnesium (HyClone Laboratories, Inc., South Logan, UT, USA). The BALF was centrifuged at 400×g for 10min at 4°C. The supernatant (BALF) volume was determined and stored at −80°C for future assay.16 The cell pellet was resuspended in minimum essential medium (MEM, Biological Industries, Kibbutz Beit, Haemek, Israel) containing 10% bovine serum albumin (Biological Industries, Kibbutz Beit, Haemek, Israel) and the final cell density was adjusted to 1×106cells/mL. The total cell count was determined with a haemocytometer using the trypan blue dye exclusion method. Cytocentrifuged preparations were stained with Liu's stain for the differential cell count. Based on standard morphological criteria, a minimum of 200 cells were counted and classified as macrophages, lymphocytes, or eosinophils.21 Differential cells are distinguished from each other based on the standard morphological criteria.22 Lymphocytes are small resting cells that are dense, inactive nucleus occupies much of the intracellular space, with occasional mitochondria. Lymphocytes are much smaller comparatively to monocytes or macrophages. Monocytes may differentiate into macrophages that are remarkably plastic to form pseudopods. Lymphocytes and monocytes/macrophages are mononuclear cells, however eosinophils, basophils and neutrophils are polymorphonuclear leukocytes because of the varying shapes of the nucleus, which is usually lobed into three segments. Neutrophils are normally found in the bloodstream although they may migrate through the blood vessels, then through interstitial tissue in a process called chemotaxis. Polymorphonuclear leukocytes are a category of white blood cells characterised by the presence of granules in their cytoplasm, distinguishing them from the mononuclear agranulocytes. Mast cells that are mononuclear cells are resident granulocytes in several types of tissues, having many granules rich in histamine and heparin. Mast cell is very similar in both appearance and function to the basophil, however they differ in that mast cells are tissue resident (particularly in mucosal tissues) while basophils are scarce (0 to 1% in white blood cells) and only found in the blood. In a usual situation, basophils, neutrophils and mast cells are relatively scarce in the BALF; therefore these cells in the BALF were not counted in this assay. Monocytes/macrophages, lymphocytes, or eosinophils in the BALF can be distinguished from each other by their characteristic morphology using light microscopy.
Assay of farnesol levels in the serum using high-performance liquid chromatography (HPLC)To analyse the farnesol level in the sera of the experimental mice, extraction was performed exactly as described in the previous study.23 An aliquot of 100μL of serum sample and 200μM farnesol standard (Sigma, St. Louis, MO, USA) were extracted with 100μL of methanol (ECHO, HPLC grade, Taiwan). After thorough mixing, the mixture was centrifuged at 12,000×g for 15min. The supernatant was collected into a 1.5mL centrifugal tube and filtered through a 0.45μm filter (Minisar SRP4, PTFE membrane, Sartorius, Goettingen, Germany). The filtrate sample was stored at −30°C until use. The solvent extraction method such as methanol or hexane, was found to be effective in extracting farnesol from a whole culture broth, reflecting a farnesol recovery about 100%.23 Therefore, we used just one single methanol extraction prior to centrifugation and collection of supernatant.
For HPLC analysis, the filtrate sample solution was ultrasonically degassed before use. The HPLC conditions were as follows: the pump (Hitachi, model L-2130, Tokyo, Japan), UV–visible detector (Hitachi, L-2400, Tokyo, Japan) and a RP-18 chromatographic separation column (4.6mm×250mm, 5μm, Tokyo, Japan). The detection was set at 210nm. The mobile phase (methanol: H2O=4: 1 (v/v), flow rate=1mL/min.) were filtered through a 0.45μm filter (Durapore, Millipore, HVLP-4700, MA, USA) and ultrasonically degassed before use.24 Quantification of farnesol levels in the sera was based on the peaks integral area ratio between the standard and sample. A mixture of E–E-farnesol isomers was selected as a standard.
Serum antibody titres1. OVA-specific IgE, IgG1 and IgG2a assayThe blood was collected into a 1.5mL vial, allowed to stand for 2h at room temperature and then centrifuged at 12,000×g for 20min at 4°C to separate the serum. The sera were collected and stored at −80°C until analysed. OVA-specific IgE, IgG2a and IgG1 were measured using an enzyme-linked immunosorbent assay (ELISA). Aliquots of 200μL per well of OVA (10μg/mL dissolved in pH 8.2, 0.1M NaHCO3 (Wako, Osaka, Japan) were added into 96-microwell plates (Nunc). The plates were incubated overnight at 4°C and then washed three times with washing buffer (0.05% Tween 20 in PBS buffer). To block non-specific binding, 200μL of blocking buffer (1% bovine serum albumin (BSA) in PBS) were added to each well. The plates were incubated at room temperature for 2h. The plates were then washed three times with washing buffer. Serum samples were diluted (1% BSA in PBS) to 1:100 for IgE, 1:2000 for IgG2a and 1:3,000,000 for IgG1 determinations before they were added to the plate wells. Aliquots of 100μL of diluted serum samples were added to the wells and then incubated at room temperature for 2h. After incubation, the plates were washed five times with washing buffer. Pooled sera from OVA-sensitised and challenged mice were also included in each assay to serve as positive controls. Biotin-conjugated rat anti-mouse IgE, IgG2a and IgG1 monoclonal antibodies (BD PharMingen, San Diego, CA, USA) were used to determine the OVA-specific antibody levels, respectively. The procedure was carried out according to the manufacturer's instructions. After incubation, the plates were washed six times with washing buffer. Aliquots of 100μL diluted (1:200) streptavidin–horseradish peroxidase (R&D Systems, Minneapolis, MN, USA) were added to the wells for 20min. After washing six times again, the plate wells were developed by adding aliquots of 100μL substrate solution containing 3,3′,5,5′-tetramethyl benzidine (TMB) (Clinical Science Products, Inc., Mansfield, MA, USA) and incubated for 20–30min in the dark. The reaction was stopped by adding an aliquot of 100μL of 2M sulphuric acid per well and the absorbance at 450nm was measured with an ELISA reader (ASYS Hitech GmbH, Austria). The plates were finally measured for absorbance at 450nm on a plate ELISA reader (Asys/Hitech Jupiter, Eugendorf, Austria). Optical densities were converted into ELISA units, where ELISA unit (E.U.)=(Asample−Ablank)/(Apositive−Ablank). ELISA unit has been widely accepted to express titres of antigen-specific antibodies because optical densities are calibrated and scientifically converted into arbitrary E.U.3,25–27
2. Assay of non-specific antibody levelsSerum total IgA, IgE, IgG and IgM antibody levels were analysed using mouse IgA, IgE, IgG and IgM ELISA kits (E90-103, E90-115, E90-131 and E90-101, respectively, Bethyl System, Montgomery, Texas, USA). The analysed protocol was as previously described.28 Serum samples were diluted to 1:500 for IgE, 1:5000 for IgA, 1:8000 for IgM and 1:35,000 for IgG detection. The plates were finally measured on an ELISA plate reader to obtain the absorbance at 450nm. The IgA, IgE, IgG and IgM antibody levels were determined using seven point standard curves.
Assay of triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c) and low-density lipoprotein cholesterol (LDL-c) levelsThe lipid levels in the experimental mouse serum were determined using TG, TC, and HDL-c assay kits (Randox Laboratories Ltd., Northern Ireland, UK), as well as a LDL-c assay kit (Fortress, Northern Ireland, UK), respectively. The lipid levels were assayed according to the manufacturer's instructions.25
Measurement of Th1/Th2 cytokine levels in BALFThe measurement of cytokine secretion levels was as previously described.29 IFN-γ, IL-2, IL-4 or IL-5 cytokine levels were determined using sandwich ELISA kits. The cytokine concentrations were assayed according to the cytokine ELISA protocol from the manufacturer's instructions (mouse DuoSet ELISA Development system, R&D Systems, Minneapolis, MN, USA). The detection limit (LOD) of the kits used in this study was <3.9pg/mL.
Statistical analysisValues are expressed as mean±SEM and analysed statistically using ANOVA followed, if justified by the statistical probability (P<0.05), by Duncan's new multiple range tests or Dunnett's test of parametric type. P<0.05 was considered significant.
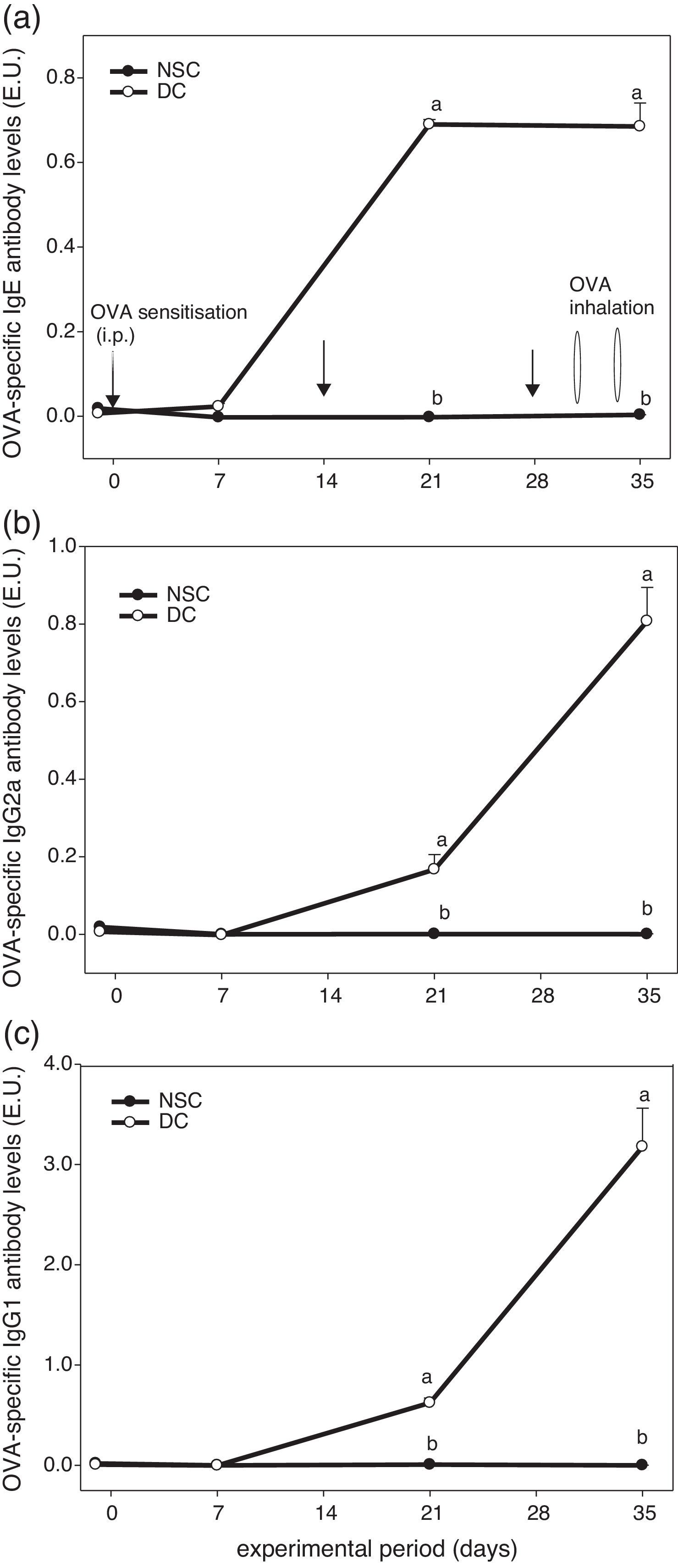
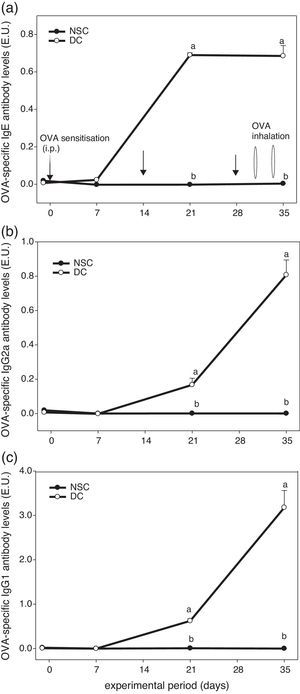
ResultsEffects of OVA sensitisation and challenge through 5 weeks on serum OVA-specific antibody titres of the experimental miceFig. 1 shows the experiment protocol and changes in serum OVA-specific IgE, IgG2a and IgG1 antibody titres of the experimental mice over a 5-week period. The results showed that serum OVA-specific IgE, IgG2a and IgG1 levels in sera of OVA-administered mice were all dramatically elevated after sensitised i.p. three times and challenged by inhalation two times. We found that OVA-specific antibody levels in the DC group were significantly (P<0.05) higher than those in the non-sensitised NSC group after Day 21. Our results evidenced that the animal model using an allergen OVA to induce allergic adaptive immune responses was successful (Fig. 1).
The experiment protocol and changes in serum OVA-specific IgE (a), IgG2a (b), and IgG1 (c) antibody titres of the experimental mice during 5 weeks experiment period. Mice were sensitised with OVA by intraperitoneal injection (i.p.) at Days 0, 14 and 28, followed by challenge with OVA inhalation at Days 31 and 34 after grouping. Values are means±SEM (n=13–15). Values at the same experimental point not sharing a common small letter are significantly different (P<0.05) from each other assayed using one-way ANOVA, followed by Duncan's new multiple range tests. E.U.=(Asample−Ablank)/(Apositive−Ablank); NSC, non-sensitised control; DC, dietary control. Serum dilution: 1:100 for IgE detection, 1:2000 for IgG2a detection and 1:3,000,000 for IgG1 detection. Some standard errors were too small to be depicted in the figure.
Levels of farnesol in sera from the experimental mice were analysed according to their HPLC chromatograms (data not shown). Table 1 shows effects of farnesol supplementation at different doses for 5 weeks on serum farnesol concentrations of OVA/AL-sensitised and challenged BALB/c asthmatic mice. The results showed that the farnesol concentrations in farnesol-administered groups increased in a dose-dependent manner, suggesting that farnesol is absorbable via the digestive tract.
Effects of farnesol supplementation with different doses for 5 weeks on serum farnesol concentrations of OVA/AL-sensitised and challenged BALB/c asthmatic mice.
| Group | Concentration (μM) |
|---|---|
| DC | ND |
| FL | 0.86±0.19 |
| FM | 1.90±0.57 |
| FH | 2.12±1.25 |
| PC | ND |
| NSC | ND |
Values are means±SEM (n=5). Data were analysed using one-way ANOVA, followed by Dunnett's test of parametric type. To perform economically, five experimental mice in each group were randomly selected to analyse their serum farnesol concentrations. There are no significant differences among farnesol-treated groups. NSC, non-sensitised control; DC, dietary control; PC, positive control (dexamethasone treatment (3mg/kg BW, 0.3mL/mouse) by gavage 2h prior to aerosolised OVA administration); FL, low dose farnesol (0.003% in AIN-76 feed); FM, medium dose farnesol (0.017% in AIN-76 feed); FH, high dose farnesol (0.067% in AIN-76 feed); ND, not detectable.
Table 2 shows effects of farnesol supplementation at different doses for 5 weeks on serum OVA-specific IgG1, IgG2a as well as IgE antibody titres of OVA/AL-sensitised and challenged BALB/c asthmatic mice. We found that serum OVA-specific IgG1, IgE (Th2 type antibody) and IgG2a (Th1 type antibody) titres in the experimental mice significantly (P<0.05) increased after OVA/AL sensitisation and challenge. Importantly, farnesol supplementation for 5 weeks exerted differential effects on serum OVA-specific antibody titres. Serum OVA-specific IgE titres dose-dependently and significantly (P<0.05) decreased, suggesting that farnesol supplementation was alleviating allergic status. In contrast, low dose farnesol supplementation (FL group) significantly (P<0.05) increased both serum IgG1 and IgG2a titres. Most importantly, further analysis on the ratio of Th1-/Th2-type antibody titres revealed that farnesol supplementation significantly (P<0.05) increased IgG2a/IgE titre ratios. Our results evidenced that farnesol supplementation ameliorated allergic status and reversed Th2-skewed immune responses in the allergic asthmatic mice via decreasing serum OVA-specific IgE titres but increasing IgG2a/IgE titre ratios. To compare farnesol's supplementation effects, DEX was selected as a positive control (PC group). Interestingly, DEX treatment significantly (P<0.05) reduced serum OVA-specific IgE titres, but could not significantly influence serum IgG2a/IgE titre ratio, indicating that DEX treatment might alleviate allergic status, but could not reverse Th1/Th2 immune responses in the experimental mice.
Effects of farnesol supplementation with different doses for 5 weeks on serum OVA-specific IgG1, IgG2a as well as IgE antibody titres of OVA/AL-sensitised and challenged BALB/c asthmatic mice.
| Group | OVA-specific antibody titres (E.U.) | Ratio | |||
|---|---|---|---|---|---|
| n | IgG1 | IgG2a | IgE | IgG2a/IgE | |
| DC | 13 | 3.18±0.38 | 0.81±0.09 | 0.68±0.06 | 1.19±0.08 |
| FL | 14 | 4.95±0.38* | 1.49±0.29* | 0.75±0.05 | 1.67±0.30 |
| FM | 12 | 3.86±0.33 | 1.39±0.19 | 0.50±0.04* | 2.87±0.35* |
| FH | 14 | 3.18±0.19 | 0.82±0.10 | 0.38±0.03* | 2.32±0.27 |
| PC | 14 | 2.53±0.17 | 0.67±0.06 | 0.47±0.04* | 1.57±0.21 |
| NSC | 15 | ND | ND | ND | |
Values are means±SEM. Values were analysed using one-way ANOVA, followed by Dunnett's test of parametric type. E.U.=(Asample−Ablank)/(Apositive−Ablank). NSC, non-sensitised control; DC, dietary control; PC, positive control (dexamethasone treatment (3mg/kg BW, 0.3mL/mouse) by gavage 2h prior to aerosolised OVA administration); FL, low dose farnesol (0.003% in AIN-76 feed); FM, medium dose farnesol (0.017% in AIN-76 feed); FH, high dose farnesol (0.067% in AIN-76 feed). Serum dilution: 1:100 for IgE detection, 1:2000 for IgG2a detection and 1:3,000,000 for IgG1 detection. ND, not detectable.
Furthermore, our results showed that OVA sensitisation and challenge significantly (P<0.05) increased serum non-specific IgE, IgA, IgM and IgG antibody titres compared to those of the non-sensitised control (NSC) group, indicating that OVA sensitisation and challenge induced inflammation in the experimental mice (Table 3). Importantly, farnesol supplementation and DEX treatment significantly (P<0.05) reduced serum non-specific IgE, IgA, IgM (Th2-type antibodies) and IgG (Th1-type antibody) levels in the OVA-administered mice, implying that either farnesol supplementation or DEX treatment may alleviate inflammation status in the experimental mice. Particularly, decreased non-specific IgE levels by farnesol might markedly mitigate allergic status in vivo. Our results suggest that farnesol supplementation may improve allergic and inflammatory status through reducing serum total antibody levels, particularly IgE. However, IgA, IgM and IgG in humoral immunity may be protective against infection. The inhibition to the production of these antibodies might slightly decrease the protective immunity in the allergic asthmatic mice.
Effects of farnesol supplementation with different doses for 5 weeks on serum non-specific IgE, IgA, IgM, and IgG antibody titres of OVA/AL-sensitised and challenged BALB/c asthmatic mice.
| Group | n | Non-specific antibody levels (μg/mL) | |||
|---|---|---|---|---|---|
| IgE | IgA | IgM | IgG | ||
| DC | 13 | 15.0±0.68 | 639±25.3 | 2442±114 | 985±50 |
| FL | 14 | 12.1±0.73* | 486±26.7* | 1765±62* | 736±30* |
| FM | 12 | 11.8±0.74* | 514±48.2* | 1999±113* | 765±38* |
| FH | 14 | 10.8±0.41* | 468±46.2* | 1704±87* | 662±43* |
| PC | 14 | 12.4±0.29* | 593±39.8 | 1928±55* | 783±30* |
| NSC | 15 | 7.36±0.18* | 479±24.9* | 1783±104* | 510±71* |
Values are means±SEM. Data were analysed using one-way ANOVA, followed by Dunnett's test of parametric type. NSC, non-sensitised control; DC, dietary control; PC, positive control (dexamethasone treatment (3mg/kg BW, 0.3mL/mouse) by gavage 2h prior to aerosolised OVA administration); FL, low dose farnesol (0.003% in AIN-76 feed); FM, medium dose farnesol (0.017% in AIN-76 feed); FH, high dose farnesol (0.067% in AIN-76 feed). Sera were appropriately diluted to 1:500 for IgE detection, 1:5000 for IgA detection, 1:8000 for IgM detection and 1:35,000 for IgG detection.
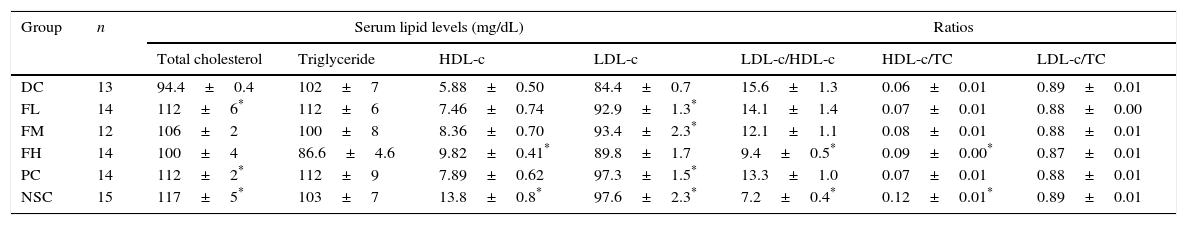
To evaluate a possible effect of farnesol supplementation on lipid profiles, lipid levels in sera of OVA-challenged mice supplemented with farnesol for 5 weeks were determined (Table 4). The results showed that TC, HDL-c and LDL-c levels in the sera of the experimental mice significantly (P<0.05) reduced after OVA sensitisation and challenge, implying that asthmatic mice may suffer cholesterol synthesis and transportation problems. OVA sensitisation and challenge did not significantly affect serum TG levels in the experimental mice, however farnesol supplementation slightly decreased (P>0.05) serum TG levels. Importantly, farnesol supplementation slightly increased TC, HDL-c and LDL-c levels. Moreover, OVA sensitisation and challenge significantly (P<0.05) increased LDL-c/HDL-c ratios, but significantly (P<0.05) decreased HDL-c/TC ratios. Farnesol supplementation significantly (P<0.05) decreased LDL-c/HDL-c ratios, but significantly (P<0.05) increased HDL-c/TC ratios dose-dependently, suggesting that farnesol supplementation improved serum lipid profiles in the OVA-sensitised and challenged mice. Interestingly, DEX treatment significantly (P<0.05) increased serum TC and LDL-c levels up close to those in normal mice (NSC group), but could not fully reverse the aberrated LDL-c/HDL-c and HDL-c/TC ratios in the sera of asthmatic mice.
Effects of farnesol supplementation with different doses for 5 weeks on serum lipid profiles of OVA/AL-sensitised and challenged BALB/c asthmatic mice.
| Group | n | Serum lipid levels (mg/dL) | Ratios | |||||
|---|---|---|---|---|---|---|---|---|
| Total cholesterol | Triglyceride | HDL-c | LDL-c | LDL-c/HDL-c | HDL-c/TC | LDL-c/TC | ||
| DC | 13 | 94.4±0.4 | 102±7 | 5.88±0.50 | 84.4±0.7 | 15.6±1.3 | 0.06±0.01 | 0.89±0.01 |
| FL | 14 | 112±6* | 112±6 | 7.46±0.74 | 92.9±1.3* | 14.1±1.4 | 0.07±0.01 | 0.88±0.00 |
| FM | 12 | 106±2 | 100±8 | 8.36±0.70 | 93.4±2.3* | 12.1±1.1 | 0.08±0.01 | 0.88±0.01 |
| FH | 14 | 100±4 | 86.6±4.6 | 9.82±0.41* | 89.8±1.7 | 9.4±0.5* | 0.09±0.00* | 0.87±0.01 |
| PC | 14 | 112±2* | 112±9 | 7.89±0.62 | 97.3±1.5* | 13.3±1.0 | 0.07±0.01 | 0.88±0.01 |
| NSC | 15 | 117±5* | 103±7 | 13.8±0.8* | 97.6±2.3* | 7.2±0.4* | 0.12±0.01* | 0.89±0.01 |
Values are means±SEM. Data were analysed using one-way ANOVA, followed by Dunnett's test of parametric type. NSC, non-sensitised control; DC, dietary control; PC, positive control (dexamethasone treatment (3mg/kg BW, 0.3mL/mouse) by gavage 2h prior to aerosolised OVA administration); FL, low dose farnesol (0.003% in AIN-76 feed); FM, medium dose farnesol (0.017% in AIN-76 feed); FH, high dose farnesol (0.067% in AIN-76 feed).
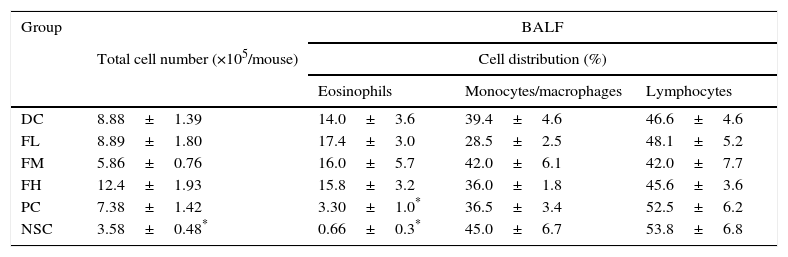
The cell number and cell distribution in BALF from the experimental mice are shown in Table 5. The results showed that OVA sensitisation and challenge significantly (P<0.05) increased total cell number (increase from 3.58±0.48 to 8.88±1.39×105cells/mouse) and the percentage of eosinophils (increase from 0.66±0.3% to 14.0±3.6%), evidencing that a mild allergic reaction in the lung and airways of the experimental mice was successfully induced. Unfortunately, farnesol supplementation did not have significant effects on infiltrations of total cells and eosinophils. Importantly, DEX treatment significantly inhibited (P<0.05) the eosinophilic infiltration in the experimental mice, suggesting potential of DEX for alleviating allergic inflammation in the lung and airways of asthmatic patients.
Effects of farnesol supplementation with different doses for 5 weeks on total cell number and differential cell distribution in BALF from OVA/AL-sensitised and challenged BALB/c asthmatic mice.
| Group | BALF | |||
|---|---|---|---|---|
| Total cell number (×105/mouse) | Cell distribution (%) | |||
| Eosinophils | Monocytes/macrophages | Lymphocytes | ||
| DC | 8.88±1.39 | 14.0±3.6 | 39.4±4.6 | 46.6±4.6 |
| FL | 8.89±1.80 | 17.4±3.0 | 28.5±2.5 | 48.1±5.2 |
| FM | 5.86±0.76 | 16.0±5.7 | 42.0±6.1 | 42.0±7.7 |
| FH | 12.4±1.93 | 15.8±3.2 | 36.0±1.8 | 45.6±3.6 |
| PC | 7.38±1.42 | 3.30±1.0* | 36.5±3.4 | 52.5±6.2 |
| NSC | 3.58±0.48* | 0.66±0.3* | 45.0±6.7 | 53.8±6.8 |
Values are means±SEM (n=9–13). Data were analysed using one-way ANOVA, followed by Dunnett's test of parametric type. Technically, a small number of BALF was not appropriately obtained in the operation procedure; thus these animals were excluded. NSC, non-sensitised control; DC, dietary control; PC, positive control (dexamethasone treatment (3mg/kg BW, 0.3mL/mouse) by gavage 2h prior to aerosolised OVA administration); FL, low dose farnesol (0.003% in AIN-76 feed); FM, medium dose farnesol (0.017% in AIN-76 feed); FH, high dose farnesol (0.067% in AIN-76 feed).
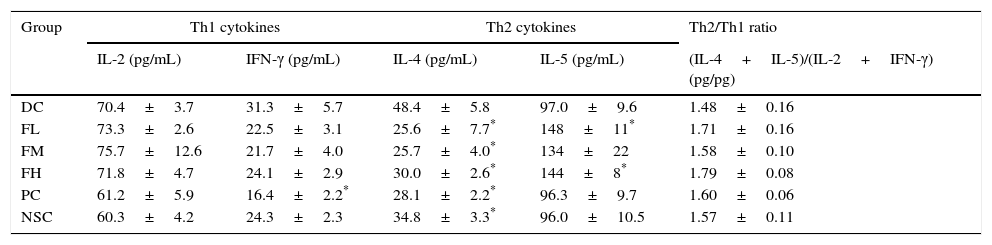
To evaluate effects of farnesol supplementation on Th1/Th2 immune balance in the lung and airways, Th1 and Th2 cytokine levels in BALF of OVA-challenged mice were measured (Table 6). The results showed that OVA sensitisation and challenge significantly (P<0.05) increased IL-4 (a Th2 cytokine) level, indicating Th2-skewed immune responses in the lung and airways of the experimental mice. Importantly, farnesol supplementation significantly (P<0.05) reduced IL-4 levels that increased due to OVA sensitisation and challenge, suggesting that farnesol supplementation may have potential to modulate Th1/Th2 immune balance towards Th1 pole in the lung and airways. Interestingly, farnesol supplementation also significantly (P<0.05) increased IL-5 (a Th2 cytokine) level, however farnesol supplementation did not significantly (P>0.05) influence the ratio of Th2/Th1 [(IL-4+IL-5)/(IL-2+IFN-γ)] cytokine levels in BALF. Although DEX treatment significantly (P<0.05) reduced both IFN-γ (a Th1 cytokine) and IL-4 (a Th2 cytokine) levels, it did not significantly (P>0.05) influence the ratio of Th2/Th1 [(IL-4+IL-5)/(IL-2+IFN-γ)] cytokine levels in BALF, suggesting that DEX may exert its anti-inflammatory effects through an immune-inhibitory property but not a regulatory property on Th1/Th2 immune balance.
Effects of farnesol supplementation with different doses for 5 weeks on Th1/Th2 cytokine levels in BALF from OVA/AL-sensitised and challenged BALB/c asthmatic mice.
| Group | Th1 cytokines | Th2 cytokines | Th2/Th1 ratio | ||
|---|---|---|---|---|---|
| IL-2 (pg/mL) | IFN-γ (pg/mL) | IL-4 (pg/mL) | IL-5 (pg/mL) | (IL-4+IL-5)/(IL-2+IFN-γ) (pg/pg) | |
| DC | 70.4±3.7 | 31.3±5.7 | 48.4±5.8 | 97.0±9.6 | 1.48±0.16 |
| FL | 73.3±2.6 | 22.5±3.1 | 25.6±7.7* | 148±11* | 1.71±0.16 |
| FM | 75.7±12.6 | 21.7±4.0 | 25.7±4.0* | 134±22 | 1.58±0.10 |
| FH | 71.8±4.7 | 24.1±2.9 | 30.0±2.6* | 144±8* | 1.79±0.08 |
| PC | 61.2±5.9 | 16.4±2.2* | 28.1±2.2* | 96.3±9.7 | 1.60±0.06 |
| NSC | 60.3±4.2 | 24.3±2.3 | 34.8±3.3* | 96.0±10.5 | 1.57±0.11 |
Values are means±SEM (n=9–13). Data were analysed using one-way ANOVA, followed by Dunnett's test of parametric type. Technically, a small number of BALF was not appropriately obtained in the operation procedure; thus these animals were excluded. NSC, non-sensitised control; DC, dietary control; PC, positive control (dexamethasone treatment (3mg/kg BW, 0.3mL/mouse) by gavage 2h prior to aerosolised OVA administration); FL, low dose farnesol (0.003% in AIN-76 feed); FM, medium dose farnesol (0.017% in AIN-76 feed); FH, high dose farnesol (0.067% in AIN-76 feed). The LOD of these kits used in this study was <3.9pg/mL.
Asthma is a Th2-skewed chronic airways inflammatory disorder with tissue eosinophilia, remodelling and altered airway function, although other T cell phenocytes including Th17, invariant natural killer (iNK)-T cells and nuocytes (non-T non-B helper T cells) were found to also contribute to the pathogenesis of asthma recently.30 Allergens play a significant role in driving allergic inflammation, although little is understood about non-allergen asthma and severe asthma in children does not have a clear Th1 or Th2 pattern.30 To establish an asthmatic mouse model in order to investigate the effects of farnesol supplementation, OVA sensitisation i.p. three times and challenge two times during a 5-week period were performed (Fig. 1). We found that serum OVA-specific IgE and IgG1 (Th2-type antibodies) levels in OVA-administered mice were all dramatically elevated after sensitisation and challenge (Fig. 1 and Table 2). Spontaneous Th2/Th1 [(IL-4+IL-5+IL-10)/(IL-2+IFN-γ)] cytokine secretion ratios by splenocytes of the experimental mice increased (data not shown). Our results evidenced that OVA sensitisation and challenge indeed modulated immune responses towards Th2 immune balance in the experimental mice. Furthermore, OVA sensitisation and challenge significantly (P<0.05) increased the total cell number (an increase of 148%) and eosinophilic infiltration (an increase of 15-folds) in BALF (Table 5). Taken together, our results proved that a mild asthmatic animal model was established and it was suitable for evaluating the effects of farnesol supplementation in vivo.18,21,25 Unfortunately, in the present study cells in BALF were identified solely by morphological criteria; examination of a minimum of 200 cells might not be accurate enough in terms of reliable identification and quantification of bronchoalveolar cell-types (Table 5). Discriminating CD4+ versus CD8+ T cells versus B cells would be desirable, as would enumeration of neutrophils. It would be far more preferable to accurately identify and enumerate different cell-types in the BALF by flow cytometry.
Among antibodies, IgE and IgG1 antibodies are generally classified as Th2-type immunoglobulins, while the IgG2a antibody is regarded as a Th1-type immunoglobulin.3 In the present study, farnesol supplementation ameliorated allergic inflammation status, via decreasing serum OVA-specific IgE titres but increasing IgG2a/IgE titre ratios (Table 2). Meanwhile, farnesol supplementation significantly inhibited serum non-specific IgE, IgA, IgM, and IgG antibody levels that were markedly increased due to OVA sensitisation and challenge, particularly total non-specific IgE levels (Table 3). Taken together, our results suggest that farnesol supplementation may moderately modulate Th2-skewed immune responses towards Th1 balance in allergic asthmatic mice.
Allergic inflammation is a Th2-skewed pattern accompanied with IL-4, IL-5 and IL-13 over-production.31 Unfortunately, farnesol could not significantly reverse Th2/Th1 [(IL-4+IL-5)/(IL-2+IFN-γ)] cytokine ratios in BALF and effectively inhibit eosinophilia in the lungs and airways (Table 6). A quite narrow set of cytokines (IFN-γ, IL-2, IL-4, and IL-5) were measured in BALF as an assay of Th1 versus Th2 immunity; a broader panel of cytokines would be preferable including IL-13 (a Th2-skewed cytokine). Navarathna et al. indicated that exogenous farnesol interfered with the normal progression of cytokine expression during candidiasis, Candida albicans, which is a commensal fungus, in a mouse model via increasing IL-5 expression.32 We also found that IL-5 levels in BALF increased as farnesol supplementation (Table 6). Increased IL-5 levels promote the differentiation, recruitment and survival of eosinophils,20 leading to a decrease in farnesol's anti-asthmatic potential in the lung and airways. Changes in serum IgE titres reflect systemically allergic inflammation responses, whereas eosinophils accumulation in the lung and airways reflects locally allergic inflammation responses. Our results showed that farnesol inhibited serum-IgE production, but failed to affect eosinophils accumulation, suggesting that farnesol supplementation might alleviate at least systemically allergic inflammation responses in the allergic asthmatic mice.
In this study TC, HDL-c and LDL-c levels in the sera of the experimental mice were significantly inhibited after OVA sensitisation and challenge, indicating the inhibition of cholesterol synthesis in asthmatic mice (Table 4). We hypothesised that some enzymes in the mevalonate pathway in the asthmatic mice were arrested, resulting in the deficiency of endogenous farnesol. However, dietary farnesol might supply the need for cholesterol synthesis in liver cells. Furthermore, dietary farnesol might facilitate the activation of farnesylated proteins. A particular farnesylated protein might be implicated in the regulation of the immune system such as allergic reactions; however this remains to be further studied. Although serum lipid profiles of mice might be dramatically changeable in different study models and treatments,33–36 in the present study farnesol supplementation markedly reversed the aberrated LDL-c/HDL-c and HDL-c/TC ratios in the sera of asthmatic mice (Table 4), indicating that farnesol supplementation might ameliorate serum lipid profiles in the OVA-sensitised and challenged mice. Meanwhile, serum farnesol levels in the experimental mice increased in a dose-dependent manner, indicating that farnesol is absorbable through the intestine (Table 1). However, serum farnesol levels were still low (2.12±1.25μM in the FH group) (Table 1). Farnesol can be produced in animal cells through the mevalonate pathway from a staring material acetyl CoA, serve as a squalene precursor, subsequently represses specifically the activity of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase in the cholesterol synthesis pathway and finally catabolised into farnesoic acid via aldehyde intermediate (farnesal) using aldo-keto reductases.37–40 We assumed that absorbed farnesol might be further metabolised into other compounds during 12h-fasting before sacrifice, however farnesol distribution in the visceral organs remains to be further studied. Importantly, farnesol can also be converted to farnesyl pyrophosphate in cells, possibly including immune cells, where it might modify signalling proteins.10 Changes in cytokine and antibody levels might be affected through this mechanism. Anti-inflammatory and anti-allergic potential of farnesol in asthmatic mice have been recently proved.41 The present study further unravel the effects of farnesol, a sesquiterpene alcohol in essential oils in many plants, on allergic and inflammatory status in ovalbumin-challenged mice.
Consequently, the present study suggests that farnesol at appropriate doses may serve as a daily supplement to improve mild allergic diseases. The most effective dose of farnesol in vivo was suggested to be low dose administration for long term. Based on our published data, daily supplementation with 50mg farnesol in humans may achieve health effects and is considerably safe.41 The present study gained some achievements. Farnesol was found to exhibit a relative Th1-inclination to normal primary immune cells in vitro.15 We assume that it has similar effects on normal mice in vivo. However, the effects of dietary farnesol on immunoglobulins and cytokines in the absence of ovalbumin challenge remain unclear and should be further studied. In addition, the effect of farnesol in the absence of ovalbumin sensitisation but with the aerosol challenge should be investigated in the future.
ConclusionsWe used OVA sensitisation and challenge to establish an asthmatic mouse model for investigating effects of farnesol supplementation on Th1/Th2 immune balance in the serum and BALF. The results showed that farnesol supplementation increased serum farnesol concentrations dose-dependently. Farnesol supplementation markedly reversed the aberrated LDL-c/HDL-c and HDL-c/TC ratios in the sera of asthmatic mice, suggesting that farnesol supplementation might ameliorate serum lipid profiles in the OVA-sensitised and challenged mice. Meanwhile, farnesol supplementation increased the ratio of serum OVA-specific IgG2a/IgE antibody levels, but reduced non-specific (total) IgE, IgA, IgM, and IgG levels that were increased due to OVA sensitisation and challenge, evidencing that farnesol supplementation might regulate Th2 immune responses towards Th1 immune balance and ameliorate inflammation in allergic asthmatic mice. Consequently, the present study suggests that farnesol at appropriate doses may serve as a daily supplement to improve mild allergic diseases and serum lipid profiles.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
This study was kindly supported by a research grant NSC101-2313-B-005-042-MY3 from the National Science Council, Taipei, Taiwan, ROC, and was supported in part by the Ministry of Education, Taiwan, ROC under the ATU plan.