The health-related quality of life (HRQoL) of asthmatic children and their caregivers is correlated to management of the disease and the presence of certain morbidity indicators. The integral assessment of paediatric asthma must include the evaluation of HRQoL among the caregivers, although existing questionnaires only partially assess the dimensions of this aspect. The present study describes a new questionnaire for evaluating HRQoL among the caregivers, comprising three dimensions (functional, emotional, and socio-occupational).
Material and methodsThe study involves two phases. A total of 81 patients between 3 and 9 years of age and their caregivers participated in the first phase, involving a qualitative and psychometric study of the preliminary version of the questionnaire (IFABI). A total of 137 patients between 2 and 17 years of age and their caregivers participated in the second phase, in which the revised version of the questionnaire (IFABI-R) was developed and subjected to psychometric evaluation.
ResultsFirst phase: The IFABI showed important reliability and internal consistency (Cronbach alpha=0.93), concurrent validity requiring improvement, and a scantly clear internal structure. Second phase: The IFABI-R showed important reliability and internal consistency (Cronbach alpha=0.90), adequate concurrent validity, and a three-dimensional structure whose three factors correspond to the three dimensions of the questionnaire.
ConclusionsThe good psychometric results obtained with the IFABI-R justify its use in paediatric asthmatic patients. The questionnaire is currently being scaled, and its sensitivity to change is being assessed.
Asthma is one of the most common health problems in children. While its clinical manifestations are similar in the different age groups, in children – particularly those aged 6–7 years – the disease presents characteristics which differ from those seen in adult patients.
According to the International Study of Asthma and Allergies in Childhood (ISAAC), between 1993 and 2002 the prevalence of asthma symptoms in Spain increased significantly in children between 6 and 7 years of age (from 6.2% to 9.4%), although it remained constant in adolescents in the 13–14 years age range (about 9.2%).1,2
The aim of treatment in childhood asthma is to secure control of the disease in order to offer better health-related quality of life (HRQoL) for both the patients and their caregivers. Treatment aims to eliminate the symptoms, maintain lung function within normal limits, reduce the schooling days lost, favour physical exercise, reduce the number of visits to the Emergency Service, and avoid the restriction of daily life activities among the patients and their parents.3
The management of childhood asthma generates important healthcare costs, since it involves regular programmed medical visits and frequent visits to the Emergency Service.4–6 Likewise, the daily life of the patients and their parents is altered, with important school absenteeism7–9 and a notorious reduction in the normal activities of the affected population.10
In addition to evaluation of the patient, the integral assessment of asthma requires the evaluation of caregiver HRQoL, since the latter is correlated to management of the disease and the presence of certain morbidity indicators: school absenteeism, reduced daily life activities, and an increased number of visits to the Emergency Service.11–16
There is no universally accepted definition for HRQoL, although the operative definitions agree that it is a multidimensional concept basically centred on the patient, and composed of four domains: physical, functional, emotional, and social. The physical domain is referred only to the patient, while the remaining three domains refer to both the patient and the caregivers.17,18
The Pediatric Asthma Caregiver’s Quality of Life Questionnaire (PACQLQ)19 is the only published questionnaire to evaluate HRQoL among the caregivers of asthmatic children.20 It comprises two domains (functional and emotional), with good psychometric properties, and has been used in the course of the present decade in a series of studies.21–25 However, we consider that the clinical usefulness of the PACQLQ is limited, since it does not contemplate the socio-occupational domain, which according to our own findings,13, 26 and those of other authors,27–29 is also altered.
The main objective of our study is to develop a questionnaire to assess the repercussions of childhood asthma upon the HRQoL of the caregivers, exploring three domains: functional, emotional, and socio-occupational. Our aim is to design an instrument with good psychomotor properties which can be used for the integral assessment of the paediatric asthmatic patient in the Primary Care setting and in Hospital Paediatric Services, as well as in research on the quality of life among the caregivers of asthmatic patients.
Material and methodsThe present study involves two phases. Phase 1: Analysis of the psychometric behaviour and qualitative assessment of the first version of the Family Impact of Childhood Bronchial Asthma Questionnaire (Cuestionario de Impacto Familiar del Asma Bronquial Infantil, IFABI), in a sample of caregivers of asthmatic children. This questionnaire was designed in the initial stage of our research. Phase 2: Design of the Revised Family Impact of Childhood Bronchial Asthma Questionnaire (Cuestionario de Impacto Familiar del Asma Bronquial Infantil Revisado, IFABI-R) and psychometric analysis of the questionnaire in a sample of caregivers of asthmatic children and adolescents.
Study samplePhase 1: Eighty-one asthmatic patients (24 girls and 57 boys) and their main caregivers (68 mothers, 11 fathers, and both parents in two cases), seen in Parc Taulí Hospital in Sabadell, and in Granollers General Hospital, Barcelona, Spain. The patient age range was 3–9 years (mean 5.78 years, standard deviation (SD) 1.87). In turn, 39.5% of the children were under 5 years of age, 34.6% were aged 5–7 years, and 25.9% were over 7 years of age. A subsample of 16 caregivers participated in the qualitative part of the study.
Phase 2: One hundred and thirty-seven asthmatic patients (56 girls and 81 boys) and their main caregivers (115 mothers, 15 fathers, both parents in three cases, and others in four cases), with a mean age of 8.27 years (SD 1.87) (range 2–17 years). A total of 54 patients were 2–6 years of age, 49 were between 7 and 11 years of age, and 34 were 12–17 years of age.
Variables and instruments- •
Severity of asthma. In both phases use was made of the childhood asthma clinical severity classification accepted in the consensus-based treatment protocols: occasional-episodic, frequent-episodic, moderate-persistent, and severe-persistent.30,31
- •
Morbidity indicators. In both cases we documented the presence of four indicators in the last three months: school absenteeism, days staying at home outside school hours, non-programmed medical visits, and number of hospital admissions.
- •
Symptoms perception. In the second phase we evaluated this variable, which has shown correlation to quality of life and family function among asthmatic children.32 We administered the PSI questionnaire,33 an experimental version in Spanish developed by our group from the Questionnaire to Measure Perceived Symptoms and Disability in Asthma.34
- •
Family impact of asthma. In phase 1 we administered the first version of the Family Impact of Childhood Bronchial Asthma Questionnaire (Cuestionario de Impacto Familiar del Asma Bronquial Infantil, IFABI), comprising 21 items with four possible responses referring to the previous three months, scored from 1–4 points, and three dimensions: functional (3 items), emotional (9 items) and socio-occupational (9 items).26 In phase 2 we designed and administered the revised version of the IFABI (Cuestionario de Impacto Familiar del Asma Bronquial Infantil Revisado, IFABI-R), comprising 15 items with four possible responses referred to the past three months, scored from 1–4 points (lesser to greater involvement), and three dimensions: functional (3 items), emotional (5 items) and socio-occupational (7 items).
We have studied the psychometric behaviour of the IFABI and IFABI-R in the respective samples:
- •
Distribution of responses. The overall score of each questionnaire was obtained by averaging the items, and the percentage of positive responses was established from the arithmetic sum of the percentages corresponding to the response options scored as 1, 2, 3 and 4 points.
- •
Dimensionality. Exploratory analysis of the principal components has been performed with varimax rotation.
- •
Reliability of internal consistency. This has been studied by analysis of internal consistency (Cronbach alpha).
- •
Concurrent validity. The relationship between severity and impact has been assessed by the Student-Fisher t-test, establishing two severity categories: episodic asthma (occasional/frequent) and persistent asthma (moderate/severe), due to the very limited number of patients with occasional-episodic asthma in both samples. The relationship between morbidity and impact was evaluated using the Mann-Whitney U-test. In turn, the relationship between parental perception of symptoms (PSI) and family impact (IFABI-R) was explored using the Pearson correlation coefficient.
The qualitative study of the IFABI comprised the assessment of content and of the level of comprehension of the items.
Participation of the patients and their caregivers was voluntary, after due explanation of the study objectives. Patients with other associated disorders were excluded from the study. The study was approved by the Ethics Committees of the respective hospital centres. The SPSS version 15.0 statistical package for Microsoft Windows was used for analysis of the data obtained.
ResultsPhase 1: Family impact of childhood bronchial asthma questionnaire, IFABIQualitative assessmentComprehension of all the items was positively rated by the 16 caregivers, though two items also received three proposals for minor changes in wording, in order to improve comprehension. The contents of 16 items were positively rated by all the caregivers, and two items moreover received three proposals for fusion; three items received 12 positive ratings; and two items received 11 positive ratings. The qualitative assessment of the questionnaire can be considered globally positive, particularly as regards comprehension of the items, with a slightly lower rating as regards content.
Psychometric study- •
Distribution of responses. The mean global score was 1.82, with a standard deviation (SD) of 0.63. All the items yielded quite heterogeneous responses; the smallest standard deviation corresponded to item 8 (SD=0.69).
- •
Study of dimensionality. The triple factor solution was found to be the most adequate, explaining 60.12% of the variance, though it fails to adjust to the three domains of the questionnaire. Only the functional items saturated in one same factor (factor 3). The emotional items preferentially saturated in factor 2, although two items saturated in factor 3, and one item in factor 1. The socio-occupational items in turn preferentially saturated in factor 1, although one item saturated in factor 2.
- •
Reliability of internal consistency. The overall reliability of the questionnaire was very high (Cronbach alpha=0.93), and all the items contributed to the adequate reliability of the instrument.
- •
Concurrent validity
- ○
Regarding severity: The mean global score and score of all the items was higher in the persistent asthma group than in the episodic asthma group, although the differences only proved significant in six items.
- ○
Regarding the morbidity indicators: A significant relationship was observed between the IFABI (global score and 13 items) and the three morbidity indicators. Likewise, five items showed a significant correlation to two indicators, and two items to one indicator.
- ○
- •
Twelve items of the IFABI meeting the following criteria have been retained:
- 1.
Qualitative assessment: Without negative content assessments.
- 2.
Factorial saturation: Saturation in the predicted factor.
- 3.
Concurrent validity: Significant correlation to one or more morbidity indicators.
- 1.
- •
Two items of the IFABI have been retained after introducing a minor change in their wording. Both items saturated in a factor different from that predicted, and we consider that modification of the wording will improve their comprehension and factorial behaviour.
- •
Two items of the IFABI were merged into a single item. These items satisfied the three inclusion requirements, although in the qualitative part of the study they received three fusion proposals due to the existence of repetitive contents.
- •
We eliminated five items of the IFABI presenting between 25% and 32% of negative qualitative content assessments. In addition, two items saturated in a factor different from that predicted, and one item was unrelated to morbidity.
The new IFABI-R questionnaire thus comprises 15 items.
Psychometric study- •
Distribution of responses
All the items yielded quite heterogeneous responses. The mean global score was 1.72 (SD=0.54), and the means of the functional, emotional, and socio-occupational domains were respectively 1.98 (SD=0.83), 1.82 (SD=0.71), and 1.60 (SD=0.54).
- •
Study of the dimensionality of the instrument
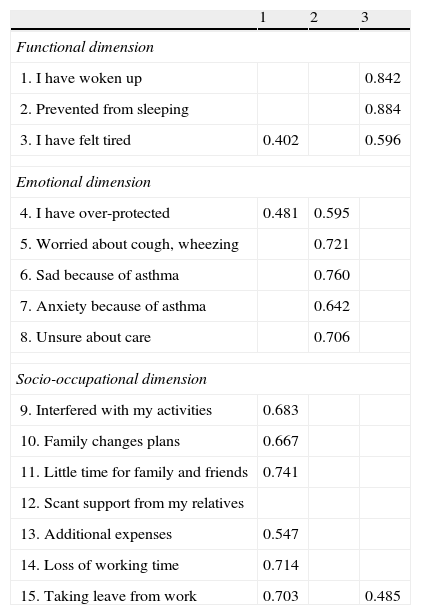
The triple factor solution was found to be the most adequate, explaining 62.15% of the variance. The functional items saturated in factor 3, the emotional items in factor 2, and the socio-occupational items in factor 1 – with the exception of a single item which failed to saturate in any factor (Table 1).
Table 1.Factorial saturations matrix obtained in the analysis of three principal components (varimax rotation) of the IFABI-R questionnaire.
1 2 3 Functional dimension 1. I have woken up 0.842 2. Prevented from sleeping 0.884 3. I have felt tired 0.402 0.596 Emotional dimension 4. I have over-protected 0.481 0.595 5. Worried about cough, wheezing 0.721 6. Sad because of asthma 0.760 7. Anxiety because of asthma 0.642 8. Unsure about care 0.706 Socio-occupational dimension 9. Interfered with my activities 0.683 10. Family changes plans 0.667 11. Little time for family and friends 0.741 12. Scant support from my relatives 13. Additional expenses 0.547 14. Loss of working time 0.714 15. Taking leave from work 0.703 0.485 Factor loadings below 0.40 have been removed from the table.
The study of the relationship between the three factors and patient age based on Pearson correlation coefficients showed the three factors to be inversely correlated to the age of the child. Accordingly, the impact of childhood asthma upon parent quality of life is seen to decrease as the child grows older (r=−0.472, r=−0.276, r=−0.500 for the functional, emotional, and socio-occupational factors, respectively).
- •
Reliability of internal consistency
The questionnaire showed important reliability: The global score showed a Cronbach alpha coefficient of 0.90, and the three factors all showed coefficients of over 0.80 (functional factor 0.85, emotional factor, 0.82 and socio-occupational factor 0.84).
- •
Concurrent validity
- ○
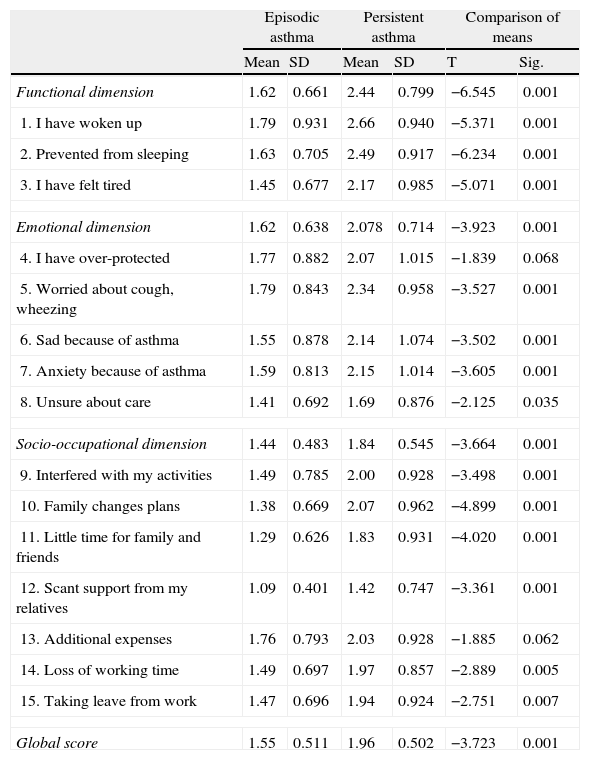
Relationship between IFABI-R and asthma severity. The global score, the scores of the three factors, and the scores of 13 items showed significantly higher mean values in the persistent asthma group than in the episodic asthma group. The remaining two items in turn showed a tendency towards statistical significance (Table 2).
Table 2.Mean and standard deviation, globally and for the items of the IFABI-R according to asthma severity. Student-Fisher t-test for evaluating differences between groups.
Episodic asthma Persistent asthma Comparison of means Mean SD Mean SD T Sig. Functional dimension 1.62 0.661 2.44 0.799 −6.545 0.001 1. I have woken up 1.79 0.931 2.66 0.940 −5.371 0.001 2. Prevented from sleeping 1.63 0.705 2.49 0.917 −6.234 0.001 3. I have felt tired 1.45 0.677 2.17 0.985 −5.071 0.001 Emotional dimension 1.62 0.638 2.078 0.714 −3.923 0.001 4. I have over-protected 1.77 0.882 2.07 1.015 −1.839 0.068 5. Worried about cough, wheezing 1.79 0.843 2.34 0.958 −3.527 0.001 6. Sad because of asthma 1.55 0.878 2.14 1.074 −3.502 0.001 7. Anxiety because of asthma 1.59 0.813 2.15 1.014 −3.605 0.001 8. Unsure about care 1.41 0.692 1.69 0.876 −2.125 0.035 Socio-occupational dimension 1.44 0.483 1.84 0.545 −3.664 0.001 9. Interfered with my activities 1.49 0.785 2.00 0.928 −3.498 0.001 10. Family changes plans 1.38 0.669 2.07 0.962 −4.899 0.001 11. Little time for family and friends 1.29 0.626 1.83 0.931 −4.020 0.001 12. Scant support from my relatives 1.09 0.401 1.42 0.747 −3.361 0.001 13. Additional expenses 1.76 0.793 2.03 0.928 −1.885 0.062 14. Loss of working time 1.49 0.697 1.97 0.857 −2.889 0.005 15. Taking leave from work 1.47 0.696 1.94 0.924 −2.751 0.007 Global score 1.55 0.511 1.96 0.502 −3.723 0.001 - ○
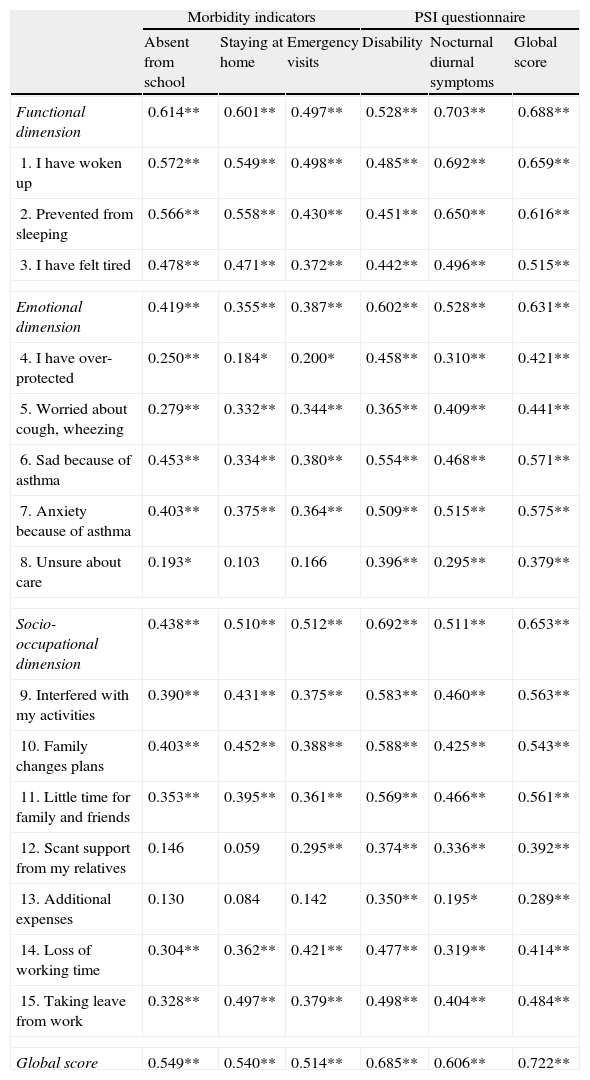
Relationship between IFABI-R and the morbidity indicators. A statistically significant relationship was observed between the three morbidity indicators and the global score, the scores of the three factors, and the scores of 13 items (Table 3).
Table 3.Correlation coefficients between IFABI-R and the morbidity indicators and PSI scores.
Morbidity indicators PSI questionnaire Absent from school Staying at home Emergency visits Disability Nocturnal diurnal symptoms Global score Functional dimension 0.614** 0.601** 0.497** 0.528** 0.703** 0.688** 1. I have woken up 0.572** 0.549** 0.498** 0.485** 0.692** 0.659** 2. Prevented from sleeping 0.566** 0.558** 0.430** 0.451** 0.650** 0.616** 3. I have felt tired 0.478** 0.471** 0.372** 0.442** 0.496** 0.515** Emotional dimension 0.419** 0.355** 0.387** 0.602** 0.528** 0.631** 4. I have over-protected 0.250** 0.184* 0.200* 0.458** 0.310** 0.421** 5. Worried about cough, wheezing 0.279** 0.332** 0.344** 0.365** 0.409** 0.441** 6. Sad because of asthma 0.453** 0.334** 0.380** 0.554** 0.468** 0.571** 7. Anxiety because of asthma 0.403** 0.375** 0.364** 0.509** 0.515** 0.575** 8. Unsure about care 0.193* 0.103 0.166 0.396** 0.295** 0.379** Socio-occupational dimension 0.438** 0.510** 0.512** 0.692** 0.511** 0.653** 9. Interfered with my activities 0.390** 0.431** 0.375** 0.583** 0.460** 0.563** 10. Family changes plans 0.403** 0.452** 0.388** 0.588** 0.425** 0.543** 11. Little time for family and friends 0.353** 0.395** 0.361** 0.569** 0.466** 0.561** 12. Scant support from my relatives 0.146 0.059 0.295** 0.374** 0.336** 0.392** 13. Additional expenses 0.130 0.084 0.142 0.350** 0.195* 0.289** 14. Loss of working time 0.304** 0.362** 0.421** 0.477** 0.319** 0.414** 15. Taking leave from work 0.328** 0.497** 0.379** 0.498** 0.404** 0.484** Global score 0.549** 0.540** 0.514** 0.685** 0.606** 0.722** * Significant correlation (p=0.05, two-sided).
** Significant correlation (p=0.01, two-sided).
- ○
Relationship between IFABI-R and parental perception of the symptoms. A significant relationship (p=0.01, two-tailed) was observed between the PSI (score of the two dimensions and global score) and the IFABI-R (global score, the scores of the three factors, and the scores of 15 items) (Table 3).
- ○
The psychometric results obtained with the revised version of the questionnaire (IFABI-R) can be considered quite satisfactory, and constitute an appreciable improvement with respect to the preliminary version of the instrument (IFABI). We highlight the following results:
- •
Dimensionality. The three-dimensional structure obtained is quite clear, with three factors corresponding to the three domains of the questionnaire; only item 12 failed to saturate in the predicted factor.
- •
Reliability of internal consistency. Important reliability of internal consistency has been obtained, with a global score presenting a Cronbach alpha coefficient of 0.90, and the three factors presenting a coefficient of over 0.80. All the items have contributed to the adequate reliability of the instrument.
- •
Concurrent validity. A statistically significant relationship has been obtained between the IFABI-R, the three morbidity indicators (school absenteeism, staying at home on holidays, and visits to the Emergency Service), the severity of asthma, and parental perception of the symptoms.
- •
Item 12 has been retained in the definitive version of the questionnaire because of the good overall psychometric behaviour of the item, and particularly its content, which evaluates the family support received by the caregiver – this variable being regarded as an important stress protection factor.
The present study reveals a strong family impact on the part of childhood asthma in the two samples evaluated, and points to the possible repercussions for caregiver health and wellbeing. These results have been described in detail elsewhere.35,36 We also emphasise that both samples showed a very large average number of days of school absenteeism, in coincidence with our earlier findings12,13 and far greater than reported in other countries.7,10 These findings are particularly relevant, since there are no data from other investigators regarding school absenteeism among asthmatic children in Spain.
The good psychometric results obtained with the IFABI-R justify its use in research and in the integral assessment of paediatric asthmatic patients. We believe that its application in clinical practice will facilitate the detection and preventive management of the asthma-related functional and psychosocial alterations that may be experienced by the caregivers of such patients – these variables in turn being responsible for increased paediatric asthma morbidity as shown in our study, and for impaired adherence to therapy. Scaling of the questionnaire and analysis of its sensitivity to change are pending issues.