INTRODUCTION
Asthma is the most common chronic illness of childhood, affecting approximately 10 % of children. Worldwide, the prevelance of childhood asthma and hospitalizations for it are increasing1.
Current therapies for the treatment of asthma in children have limitations for example requiring inhalations, multiple daily administrations or plasma drug level monitoring2. For this reason new therapies that are effective, well-tolerated, and easily administered should be used in the treatment of asthma in childhood.
Leukotrienes, known mediators of asthma, affect the airways by decreasing ciliary activity, increasing mucous secretion, increasing vascular permeability and promoting eosinophil migration in to airways mucosa3. Cysteinyl leukotrienes (LTC4, D4, E4) are potent bronchoconstrictors4. The role of leukotrienes in the pathogenesis of asthma has been demonstrated in investigational studies with compounds that block the effect of leukotrienes. Studies of montelukast, a leukotriene receptor antagonist, have demonstrated objective and subjective improvement in asthmatic patients aged 6 years and older5,6. Also a protection have demonstrated, during 24 hours from bronchoconstriction inducted by exercise and LTD47,8. In a study examining 6 to 14 year old chronic asthmatic children, montelukast sodium has been found to be an effective and nontoxic therapy5.
The purpose of this study was to determine the clinical effects, tolerability, reliability of montelukast and compare with inhaled corticosteroids. It is also aimed for both drugs, to determine their effects on urinary LTE4 level.
MATERIALS AND METHODS
This study was randomized, 2-period, 14 weeks, prospective parallel group study. The study consisted of 2 week run-in period and 12 weeks active treatment period. 8-14 years old outpatients with a history of mild persistent asthma, clinically stable and with FEV1 higher than 80 % of the predicted value were enrolled.
Study exclusion criterias included active upper respiratory tract infection within 3 weeks, acute sinus disease requiring antibiotic treatment within 1 week, emergency treatment for asthma within 1 month. Excluded medications included astemizole within 3 months; oral or parenteral corticosteroids within 1 month; cromolyn, nedocromil, oral or long-acting β-agonist, antimuscarinics, cimetidine, metoclopramide, phenobarbital, phenytoin, terfenadine, loratadine or anticholinergic agents within 2 weeks; and theophylline within 1 week before the prestudy visit.
Asthma symptom scores, peakflowmeter measurements and β -agonist usage were determined in 2 week run-in period. Patients were divided 3 groups randomly. In group 1, 5 mg chewable montelukast tablet administered once daily in the evening, in group 2, 400 mcg inhaled budesonide twice daily and in group 3, both drugs were administered together.
Physical examinations and spirometry measurements were performed between 6 and 9 AM. Patients receiving scheduled concomitant inhaled corticosteroids withheld their morning dose until completion of a clinic visit. Short acting β-agonists and montelukast were withheld for at least 6-10 hrs respectively, prior to spirometry. Spirometry measurements were collected with a standart spirometer (sensor medics V max 20 series, 2130 spirometer V6200 Autobox) at each visit. The largest FEV1 from a set of 3 acceptable maneuvers at each visit was recorded as the true value. Airway reversibilty was tested at each period.
A daily diary card which contains daytime asthma symptoms, nighttime awakening scales, PEF measurements, needed β-agonist use, the use of oral corticosteroids rescue, an unscheduled visit to a doctor's office or hospitalization due to worsening of asthma, exercise capacity, moods and condition of elimination was used in the study. At the prestudy visit, patients received a peakflowmeter (Ferraris pocket peakflowmeter). The PEF was measured by the patient in the morning upon arising and in the evening at bedtime before taking study medication. In each visit, clinical improvement, exercise tolerance, moods and elimination status were recorded and symptom scores were formed. On completion of 12 weeks period, physicians and patients independently evaluated the change in the patient's asthma (global evaluations) by selecting the most appropriate response using a 7-point scale (very much better 0, moderately better, a little better, unchanged, a little worse, moderately worse, very much worse 6). Nocturnal awakening was recorded at each visit. No awakening was recorded "0", one time in two weeks was "1", one time in a week was "2" and greater than 2 times in a week was "3".
An increase of more than 70 % from baseline in β-agonist use, an increase of inhaled corticosteroid usage, worsening of asthma requiring oral corticosteroid rescue, "awake all night" with asthma, an increase more than 50 % from baseline in symptom score, a decrease of more than 20 % from baseline in PEF, unsheduled visit to a doctor's office or hospitalization and irregular drug usage resulted in discontinuation.
Laboratory tests (hematology, biochemistry, urinanalysis, urinary LTE4 level), electrocardiography and epidermal skin prick tests were obtained at the beginning of 1st period and at the end of the 2nd period. Epidermal skin prick tests were done with common allergens to investigate the atopy. House dust mites, grass, tree, cereal, wild grass pollens, animal danders, moulds, cacao, cockhroach and latex sensitization were evaluated with allergopharma prick tests. Skin reactions were compared with histamine (positive control) and saline (negative control). Allergens and positive-negative controls were applied to volar surface of arm epidermally. Skin reactions were evaluated with the method of Aas and Belin after 15 minutes9. Hematology and biochemistry tests, total IgE measurements and eosinophil counts were done prestudy and after 12 weeks treatment period. Hematology assesments and eosinophil counts were analysed by an automated cell counter (Beckman coulter, Gen-S model). Biochemical parameters were analysed by Hitachi 747-200 equipment with Sigma kits. Total IgE measurements were done nephalometrically by Dade Behring equipment. 12-lead electrocardiograms were performed by Autocardiofax equipment.
Urine samples were taken pre and post-treatment in order to measure urinary LTE4 level and the effects of the treatment on the urinary output level of LTE4, by acidifying to pH 3 with 6N hydrochloric acid (HCL) and stored at 70 °C till analysis. All glass equipments used in this study were acidified and pure water was used. In order to eliminate changes occuring in urine volume, creatinine was measured by autoanalyzer using Jaffe method. Urine samples were purified before enzyme immunoassay (EIA) measurement and studied by the principle of EIA with compatition. In the purified samples LTE4 levels were determined by using LTE4 EIA kit (Cayman Chemical Company, Ann Arbor, USA). In this method when LTE4 concentration was fixed, free-LTE4 level was variable.
STATISTICAL METHODS
The statistical analysis were calculated by SPSS 8.0 statistical programme. For multiple group avarage comparison Kruskal-Wallis-One-Way Anova, one of the non-parametric statistic methods was used. For double group avarage comparison Mann-Whitney U test was used. For the comparison group rates Pearson chi-square test or Fisher absolute chi-square test was used. For the dependent group comparison Friedman and double group comparison Wilcoxon Signed Rank Test was used. Statistical significance was accepted as p < 0.05.
RESULTS
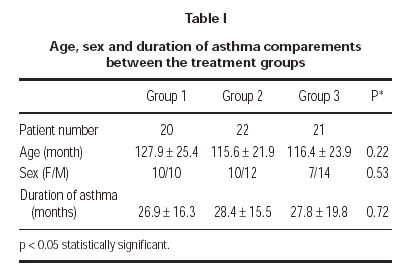
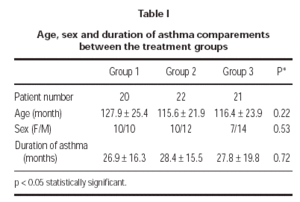
A total of 63 patients (27 female and 36 male) were enrolled in the study. There were no clinically significant differences between 3 treatment groups in their baseline characteristics (table I). 3 patients could not be reached for the follow-up; 1 patient (4.5 %) in group 1 and 2 patients (9.5 %) in group 3. Two patients (9.1 %) in group 2 was discontinued because of pneumonia. One patient (4.7 %) in group 3 discontinued because of asthma attack. 57 patients (19 patients in group 1, 20 patients in group 2 and 18 patients in group 3) completed the study.
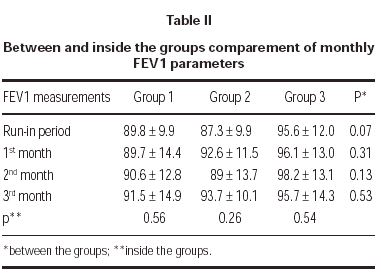
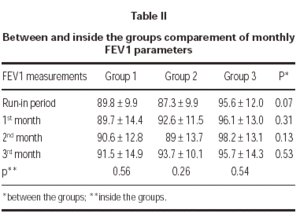
FEV1 increased in each tereatment groups compared to run-in period but there was no statistically significant difference between groups. Also there was no statistically significant difference in a group between the visits (table II).
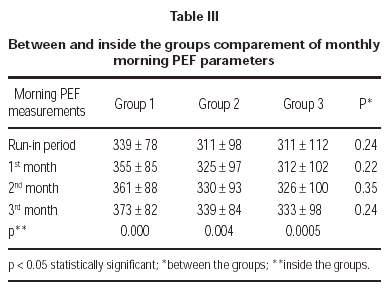
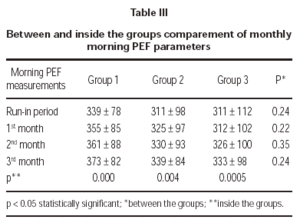
Three treatment groups demonstrated significant improvements in morning PEF measurements (table III).
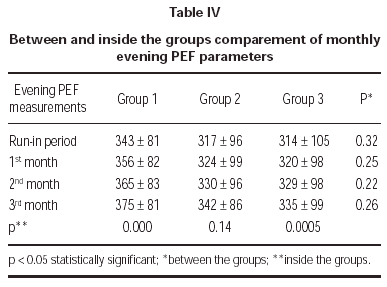
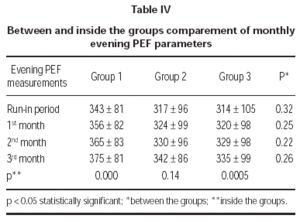
Evening PEF measurements demonstrated no statistically significant difference between treatment groups (table IV).
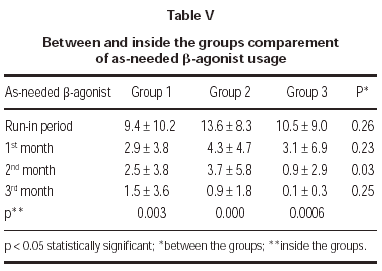
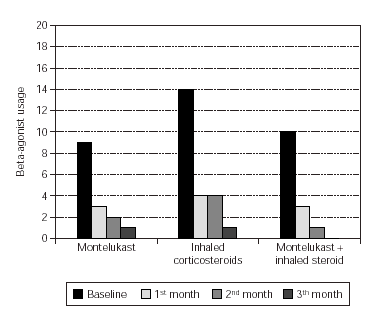
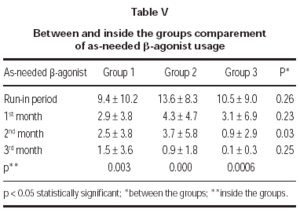
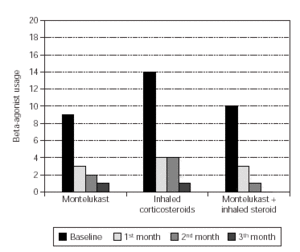
Total monthly as-needed β -agonist use was decreased statistically significant in all 3 groups (p = 0.003, p = 0.000, p = 0.0006, respectively). Evaluation of β -agonist usage between the groups showed statistically significant decrease in 2 months and also significant decrease was shown in the 3rd group when compared with the other groups (p = 0.003) (table V and figure 1).
Figure 1.--Beta-agonist usage in three groups.
Symptom scores were formed by clinical improvement, exercise tolerance, elimination adaptation, patients' mood and doctors' observations. There was no statistically significant differences between the groups when comparing the symptom scores. Inside group evaluations in the 1st month showed statistically significant clinical improvement in all three groups (patients' evaluation) (p = 0.002, p = 0.01 and p = 0.001, respectively). According to doctors' evaluation, clinical improvement was not statistically significant but numerically favored. Exercise capacity improvement evaluated by doctors and also by patients was detected in all of the 3 groups but only 3rd group showed statistically significant increase (only by patients' evaluation) (p = 0.03). There were no statistically significant difference between and inside the groups in elimination adaptation and mood variations which were evaluated by the patients and doctors. Discontinuations because of worsening of asthma, rescue oral corticosteroid use, asthma control days, nocturnal awakening did not reach a statistically significant level.
In laboratory evaluations; there were no statistically significant differences in hematologic parameters. Neutrophil count was statistically significantly decreased in group 1 but this decrease value was in the normal range of neutrophil count (p = 0.03). Statistically significant decrease was not detected in the peripheral blood eosinophil counts in group1 but statistically significant decrease was detected in group 2 (p = 0.03). There was no statistically significant difference between treatment groups when examining the biochemical parameters and total IgE values after treatment. There was statistically significant difference in direct biluribine level in group 3 after the treatment. But the value was not outside the normal range (before and after treatment; 0.17 ± 0.09 and 0.24 ± 0.08, respectively) (p = 0.02). In 3rd group there was statistically significant increase in the value of patients serum transaminase level, but this level was not pathologic (before and after treatment; 14 ± 3.4 and 16.2 ± 3.5, respectively) (p = 0.046). No patient discontiued the treatment because of laboratory abnormality.
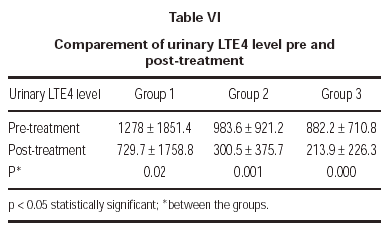
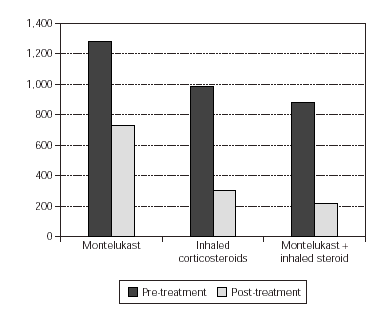
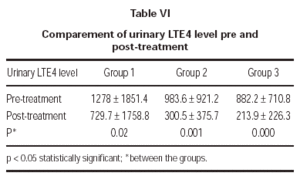
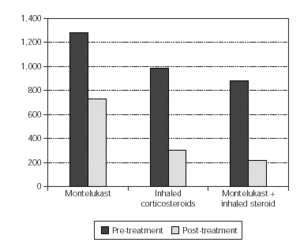
In the evaluation of urinary LTE4 level, there was statistically significant decrease in all 3 groups (p = 0.02, p = 0.001, p = 0.000, respectively). There were no statistically significant differences in urinary LTE4 levels before and after treatment (table VI and figure 2).
Figure 2.--Comparement of urinary LTE4 level pre and post-treatment.
There were no significant differences between the active treatment period and run-in period in the frequency of any adverse experience. The most common adverse experience were headache, abdominal pain and upper respiratory tract infection. There was no significant difference between the treatment groups in side-effect profile. Two patients (9.1 %) in group 2 were discontinued because of pneumonia and 1 patient (4.7 %) in group 3 was discontiued because of asthma attack.
DISCUSSION
This study demonstrated the therapeutic benefit of montelukast in 8 to 14 years old patients with mild persistent asthma after a 12 week treatment period. Montelukast treatment demonstrated significant improvements as much as single inhaled corticosteroid treatment and inhaled corticosteroid plus montelukast treatment. Montelukast, compared with inhaled corticosteroid, demonstrated similar improvements in FEV1 and PEF measurements, total daily as-needed βagonist use and asthma symptom scores. Inhaled corticosteroids demonstrated statistically significant decrease in peripheral blood eosinophil count.
There was no evidence of tolerance in this 12 week treatment period. It was notified that in some studies of leukotriene receptor antagonists, tolerance problem was occured10, but there was no literature on tolerance with the usage of montelukast sodium11.
Asthma is the most common chronic illness of childhood, affecting school attandence1. Asthma affects the physical, social and emotional aspects of the lives of children12 Montelukast, compared with other 2 treatments, demonstrated no statistically significant improvement in symptom score. The eosinophil is an inflammatory effector cell that plays a critical role in the pathogenesis of asthma. This cell and its mediators are found in increased quantities in bronchial tissue and associated with asthma severity13. Peripheral blood eosinophil count may show airway eosinophil count indirectly. Montelukast and inhaled corticosteroids have been shown to affect similarly on the peripheral blood eosinophil counts in asthmatic patients14. Corticosteroids increase eosinophil apoptosis15 and leukotriene receptor antagonists decrease eosinophil maturation in bone marrow16,17. In our study, montelukast demonstrated no significant difference in eosinophil count but inhaled corticosteroids demonstrated statistically significant decrease.
The rate of clinical adverse experiences between the montelukast and placebo controlled studies and inhaled corticosteroid compared studies were all similar18,19. In this study there was no significant difference between the montelukast and inhaled corticosteroid treatment groups in the frequency of any adverse experiences. Montelukast, 5-250 mg/day dosage, was well tolerated and demonstrated a safety profile biochemically and hematologically7,18. Laboratory adverse experiences were infrequent, mild, transient and similar between the montelukast, inhaled corticosteroid and inhaled corticosteroid plus montelukast groups. Montelukast sodium treatment for the patients who have limited leucocyte count and inhaled corticosteroid plus montelukast sodium treatment for the patients who have abnormal liver function tests should be used carefully. This study demonstrated that white blood cell count and serum transaminase levels have to be followed during treatment period.
Total IgE levels were not statistically significant but numerically decreased in all 3 treatment groups, so this parameter is not appropriate for the disease follow-up. There is no study about IgE and montelukast treatment relation in the literature. Also there is no literature about allergen sensitivity. In this study we demonstrated no relation between the treatment protocols and allergen sensitivity. Overall, the results of this study suggest that montelukast would be effective in allergic and non-allergic bronchial asthma, and would not have influence the skin prick test results.
In our study we examined the urinary LTE4 level as a marker of airway inflammation in chronic asthma, and its variations with the antiinflammatory drugs such as inhaled corticosteroids and montelukast. LTE4 is the major metabolite of leukotriene metabolism, it is excreted in urine and is more stable when compared with other leukotrienes. Due to this fact urinary LTE4 level was used as measurement of leukotriene production in human being20. It was determined that urinary LTE4 outflow did not demonstrate diurnal variation and in healthy human beings urinary LTE4 outflow would be between 0-458 pg/ml creatinine21. Pre-treatment urinary LTE4 levels of our patients were found to be higher than the LTE4 urinary values of healthy human beings determined in literature.In all three groups, there was statistically significant decrease in urinary LTE4 outflow after the treatment. This result supports that montelukast sodium has great a effect on inflamatory progress in asthma, as inhaled corticosteroids.
In literature, it was mentioned in vitro that cycteinyl leukotriene production was depressed by corticosteroids, but in vivo studies on asthmatic patients show that in spide of oral corticosteroid administration, cycteinyl leukotrienes in airway exist22. As a result anti-leukotrienes supply new dimentions to the asthma treatment. In this study it is demonstrated that single usage of montelukast sodium in mild persitent asthma is an easy and safe method and it is as effective as inhaled corticosteroids or inhaled corticosteroids plus montelukast sodium. The place of montelukast sodium in asthma treatment would be more clear by the increasing number of studies examining this drug.