The safety and efficacy of sublingual immunotherapy (SLIT) have been confirmed by many studies. However, in China, the research on efficacy and safety in young and older children with allergic rhinitis (AR) is still rare.
ObjectiveThe aim of this retrospective study is to evaluate the efficacy and safety of SLIT with Dermatophagoides farinae drops in pre-school and school-age children with AR.
MethodsA total of 282 subjects aged 2–13 years with AR received a two-year course of sublingual immunotherapy along with pharmacotherapy. According to the age, patients were defined as the pre-school group (2–6 years old, n=116) and school-age group (7–13 years old, n=166). Total nasal rhinitis symptom scores (TNSS), visual analogue score (VAS) and total medication scores (TMS) were evaluated at four time points: baseline, after SLIT for half a year, one year and two years. The adverse events (AEs) were evaluated at each visit.
ResultsAfter two-year SLIT, the four rhinitis symptom scores, TNSS, VAS and TMS scores were significantly lower than baseline (all P<0.05). The comparison of efficacy between one and two-year duration showed no significant difference in global clinical outcomes (all P>0.05). In addition, there were no significant differences between the pre-school and school-age group in TNSS (all P>0.05), VAS (all P>0.05) and TMS scores (P>0.05) after SLIT for half a year, one year and two years. No severe systemic AEs were reported.
ConclusionSLIT with D. farinae drops is clinically effective and safe in pre-school and school-age patients with house dust mites (HDMs)-induced AR.
Allergic rhinitis (AR) is characterised by excessive eosinophil infiltration, Th2 cytokine responses, elevated levels of allergen-specific IgE, mucus secretion and airway hyperresponsiveness.1 In China, the prevalence of AR in children has increased from 9.1% in 20012 to 15.4% in 2010,3 and house-dust mites (HDMs) have been documented to be the most prevalent allergens.4 Symptomatic treatment is based on antihistamines and corticosteroids. Apart from eliminating allergens, which can be difficult, allergen specific immunotherapy (ASIT) is at present the only aetiological treatment able to alter disease progression.5,6
The latest position paper document ASIT has practically no controversy in the treatment of AR and allergic asthma.7 And in the latest national guidelines for the diagnosis and treatment of AR, it is suggested that ASIT should be used as first-line treatment for AR.8 As a new route of administration of ASIT, the safety and efficacy of sublingual immunotherapy (SLIT) have been confirmed by meta-analysis and systematic review.9–11 However, in China, the research on efficacy and safety of SLIT in young children and older children is still rare.
Materials and methodsSubjectsIn total, 282 patients aged 2–13 years were involved from December 2012 to March 2014. The inclusion criteria were: (1) diagnosed with moderate-to-severe/persistent AR; (2) allergic symptoms and signs such as runny nose, itchy nose, sneezing, nasal crease, pale and swollen nasal mucosa, and watery discharge; (3) positive clinical history of HDM allergy and positive skin-prick test (SPT) for D. farinae and serum-specific immunoglobulin E (IgE) ≥0.7KU/L. The exclusion criteria included: (1) patients with immunodeficiency; (2) with bronchial asthma, acute respiratory infections and other diseases that may cause respiratory symptoms; and (3) patients having received allergen-specific immunotherapy previously.
The study was approved by the ethics committee of the Beijing Children's Hospital, Capital Medical University. We obtained informed consent from all the patients.
SLIT with HDM extractAll patients were treated with sublingual immunotherapy by Der. f extracts (CHANLLERGEN, Zhejiang Wolwo Bio-Pharmaceutical Co., Ltd., Huzhou, Zhejiang, China) in this study, which were officially approved by the Chinese Food and Drug Administration in 2006. The biologically standardised extracts were labelled in concentration of total protein and used in the form of drops (No. 1 1μg/mL; No. 2 10μg/mL; No. 3 100μg/mL and No. 4 333μg/mL). Subjects were instructed to take increasing dose from No. 1 to No. 3 during the first three weeks. 1, 2, 3, 4, 6, 8, 10 drops were given day after day in a week, respectively. Children under 14 years old took three drops of No. 4 solution daily in the maintenance therapy phase from the fourth week. The first dose taken was required to be performed under medical supervision. Subjects were instructed to self-administer at home daily and keep the drops under the tongue for 1–3min, then swallow and not to drink for 15min. Guardians of the young children were asked to help administer the doses. In case of adverse events, the dosage increase was properly delayed or reduced under the guidance of physicians.
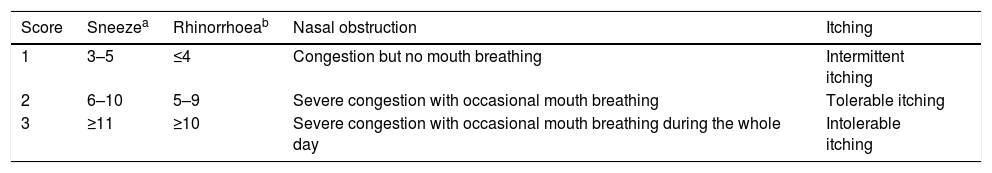
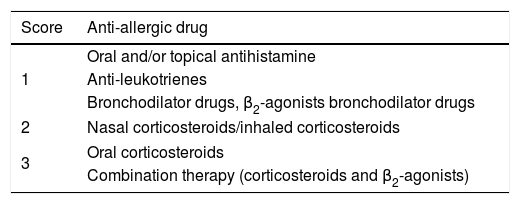
Symptoms, medication scoring system and visual analogue scaleBefore the treatment, nasal symptoms (nasal discharge, nasal obstruction, itching, sneezing), medication use and overall severity of symptoms of patients were recorded as baseline values in files. During the treatment, patients were asked to accept follow-up visit in hospital or by telephone every six months. The total nasal symptoms score (TNSS), total medication score (TMS) and visual analogue score (VAS) of subjects were evaluated by physicians at each visit. TNSS was the sum of four nasal symptoms scores. These nasal symptoms were evaluated according to a 0–3 point scoring system12 (Table 1). TMS was evaluated as 0–3 points according to the medicine use8 (Table 2). VAS represents the overall severity of symptoms through a 10-cm visual analogue scale.13 A 10-point scoring system was used for assessing subjective symptoms. 0 points indicated “no symptom”, 10 points indicated “extremely severe symptom”.
Nasal symptom scores.
All of the AEs reported during the study period were recorded on a diary card, as were aggravating local reactions/moderate reactions, drug therapy or other treatments as needs and/or proper delay or reducing of SLIT dose. AEs were rated on five levels (0–4 scale) according to the grading system proposed by the European Academy of Allergy and Clinical Immunology,14 which is based on the rate of onset and severity of the reactions.
Statistical analysisThe statistical analysis was performed with SPSS version 20.0 software (SPSS, Inc., Chicago, IL, USA). The statistical significance of difference was determined by the non-parametric Mann–Whitney U test or Wilcoxon signed rank test. P<0.05 (*) represented significant difference.
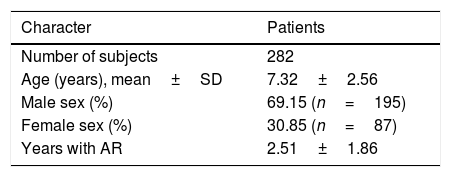
ResultsPopulation characteristicsA total of 282 subjects aged 2–13 years old were involved in this study. Baseline characteristics of 282 subjects were reported before treatment. Age, sex ratio and course of disease were recorded and are shown in Table 3.
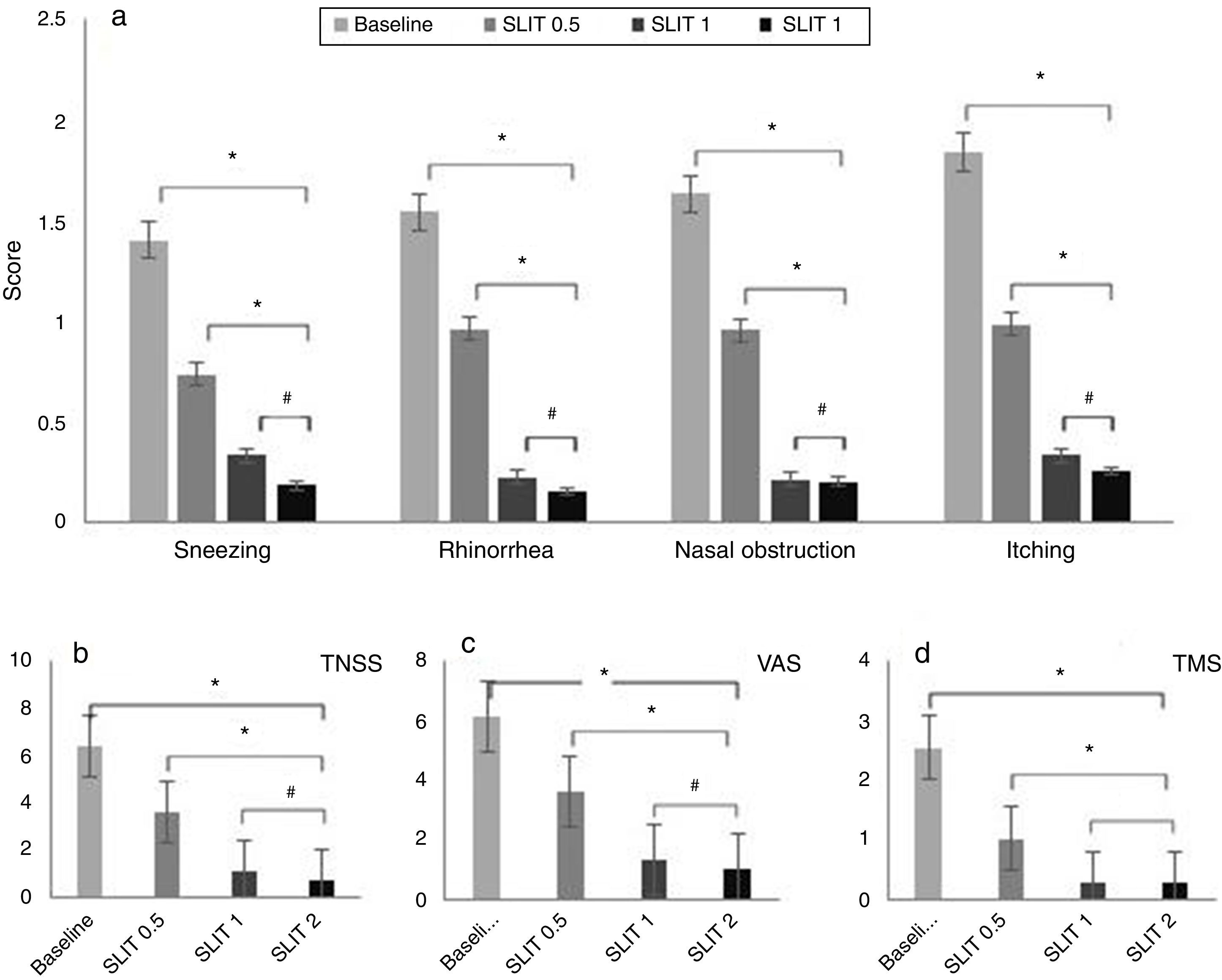
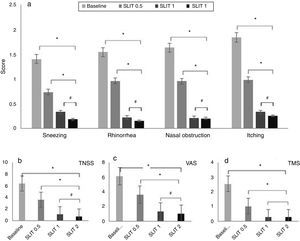
Effects on rhinitis symptom scores, TMS and VASAfter two-year SLIT, the four rhinitis symptom scores, TNSS, VAS and TMS scores were significantly lower than baseline (Sneezing: Z=16.243; Rhinorrhoea: Z=16.488; Nasal obstruction: Z=17.450; Itching: Z=17.138; TNSS: Z=21.657; VAS: Z=21.690; TMS: Z=20.108, all P<0.05).
After two-year SLIT, compared with half one year duration, all scores in patients were significantly decreased (Sneezing: Z=6.996; Rhinorrhoea: Z=9.752; Nasal obstruction: Z=9.426; Itching: Z=9.459; TNSS: Z=13.373; VAS: Z=12.231; TMS: Z=8.382, all P<0.05).
The comparison of efficacy between one and two-year duration showed no significant difference in global clinical outcomes (Sneezing: Z=2.202; Rhinorrhoea: Z=1.027; Nasal obstruction: Z=0.130; Itching: Z=0.719; TNSS: Z=2.022; VAS: Z=2.348; TMS: Z=0.147, all P>0.05) (Fig. 1a–d).
Four rhinitis symptom scores, TNSS, VAS and TMS scores before and after SLIT treatment. (a) Four rhinitis symptom scores, (b) TNSS, (c) VAS, and (d) TMS. There was a significant decline in four rhinitis symptom scores, TNSS, VAS and TMS scores after treatment (*P<0.05, #P>0.05, compared with the baseline value). SLIT=sublingual immunotherapy; TNSS=total nasal symptom score; VAS=visual analogue scale; TMS=total medication score.
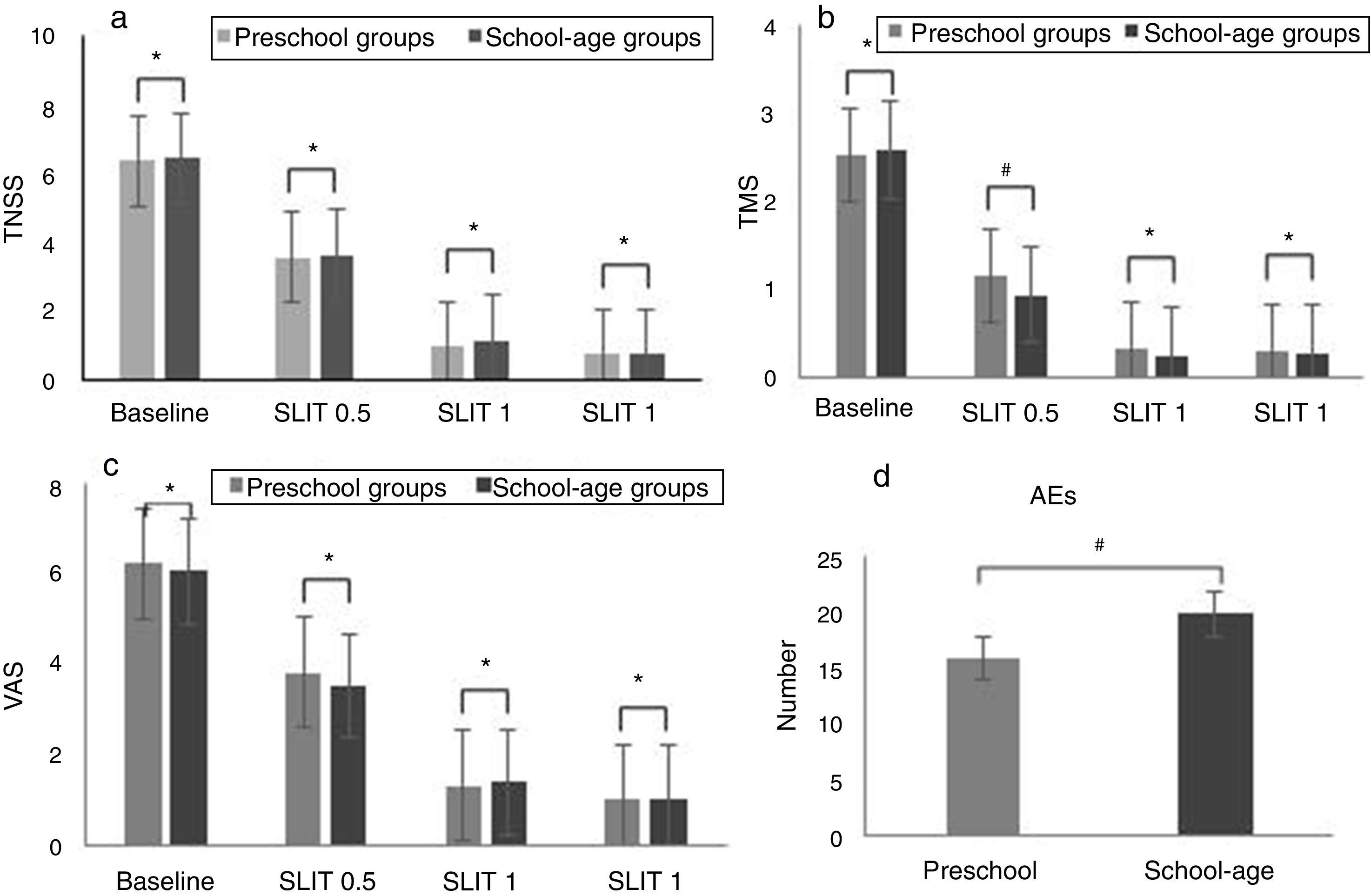
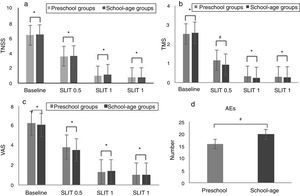
In addition, there were no significant differences between the pre-school group and school-age group in TNSS (Baseline: Z=0.053; SLIT 0.5: Z=0.010; SLIT 1: Z=2.132; SLIT 2: Z=0.020, all P>0.05), VAS (Baseline: Z=0.689; SLIT 0.5: Z=2.555; SLIT 1: Z=0.209; SLIT 2: Z=0.001, all P>0.05) and TMS scores (Baseline: Z=0.522; SLIT 1: Z=1.468; SLIT 2: Z=0.267, all P>0.05) after SLIT for half a year, one year and two years (Fig. 2a–c).
TNSS, VAS and TMS scores in pre-school and school-age groups before and after SLIT treatment. (a) TNSS, (b) TMS, (c) VAS (*P<0.05, #P>0.05, compared between two subgroups). SLIT=sublingual immunotherapy; TNSS=total nasal symptom score; VAS=visual analogue scale; TMS=total medication score; AEs=adverse events.
None of the children required hospitalisation or withdrew from the study because of AEs. No severe systemic AEs, anaphylaxis, or use of adrenaline were reported. 30 patients reported 36 AEs. Sixteen and 20 AEs were found in the pre-school group and school-age group, respectively. The majority of AEs were slight local reactions and all AEs were relieved. There were no significant differences between the pre-school group and school-age group (χ2=0.063, P>0.05) (Fig. 2d).
DiscussionIn a randomised double-blind placebo controlled study, Bahceciler et al.15 found that half-a-year SLIT can obviously improve the lung function of AR with allergic asthma (AS) in children, and reduce inhaled corticosteroids. A study of 3–13 year old children with AR for one year of SLIT, found that after treatment for seven months, compared with the control group, SLIT group has sustained efficacy during the treatment period.16 A large number of research data showed that SLIT can significantly reduce the severity of symptoms in AR. The effect is closely related to the course of SLIT, but related research is still rare. In this study, we found that the subjective symptoms decreased significantly after SLIT for six months. In addition, there are no significant differences between the one- and two-year SLIT groups. The efficacy has sustained during the whole SLIT period, and the symptoms gradually stabilised after one year.
Dust mite is one of the most common indoor allergens and the main cause of AR in children. In daily life, especially for children, it is very difficult to avoid exposure to dust mites, so the early intervention of AR caused by dust mites should be paid enough attention.17 Shao et al.16 found that SLIT with D. farinae extracts is effective in subjective and objective symptom improvements in children aged 3–5 years. But in China, there are few reports about SLIT in children with AR, especially in young children. In this study, 282 children with dust mite AR were divided into pre-school (2–6 years old) and school-age (7–13 years old) groups, and the results showed that there were no significant differences in TNSS, VAS and TMS scores between these two groups after SLIT for half a year, one year and two years. It is worth mentioning that 15 children aged 2–3 years were included in our study, and the symptoms have been greatly improved after two-year SLIT.
During the course of treatment, 30 patients reported 36 adverse reactions, the total incidence of adverse reactions was only 10%. Moreover, the majority of AEs were slight local reactions. These AEs generally occurred during the first few weeks of treatment and were relieved within a few days without any medical intervention. Other AEs were relieved after dose adjustment of D. farinae extracts along with medication (oral antihistamines or intranasal corticosteroid) according to the suggestions from physician.
ConclusionSLIT with D. farinae drops is clinically effective and safe in pre-school and school-age patients with HDM-induced AR, including very young children less than four years old.
Conflict of interestThe authors have no conflict of interest to declare.
This work was supported by Beijing Municipal Science and Technology Project (Z131100006813044) and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201508). We thank the staffs from Beijing Children's Hospital for their help and collaboration.