In recent years, traditional diets enriched with fresh plant-based foods have been gradually abandoned, increasing the consumption of animal foods and highly processed food. The aim of this study was to assess the effects of a nutritional intervention with a Traditional Mediterranean Diet in patients with recurring colds (RC) and frequent inflammatory complications (IC).
MethodsProspective before-after comparison study of 63 girls and 65 boys aged 1–5 years were included over a year in the nutritional programme “Learning to eat from the Mediterranean”. We studied clinical and therapeutic variables and various anthropometric parameters.
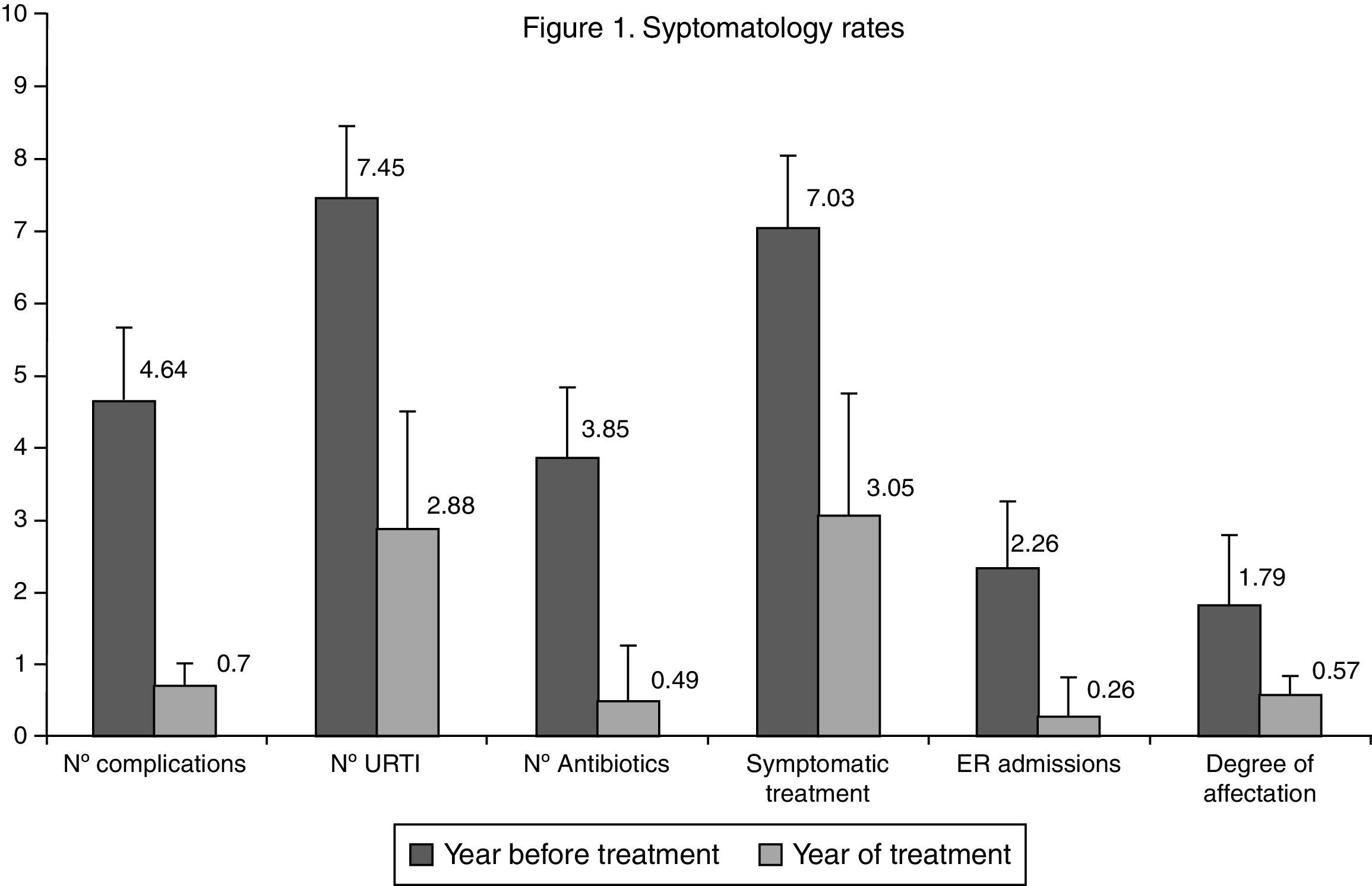
ResultsAll the studied indicators (number of catarrhal episodes CB, degree of intensity, emergency and hospital admissions) showed a positive and statistically significant evolution, evidenced from the first weeks of starting treatment, until the end of the year, after which 53.9% of patients had no CB, 25% had only one, and 16.4% had two episodes, compared to the 4.64 episodes on average in the previous year. Antibiotic use decreased by 87.4%, from 3.85±1.27 times/patient/year to 0.49±0.79 (p<0.001). Symptomatic treatment decreased by 56.7%, from 7.03±2.76 to 3.05±1.69 (p<0.001). The satisfaction of the families was very high. The Kidmed index, which assesses the quality of the Mediterranean Diet, increased from 7.8 to 10.9 points.
ConclusionThe adoption of a Traditional Mediterranean Diet could be a major contribution to the improvement of patients with recurring colds and frequent inflammatory complications.
Upper Respiratory Tract Infections (URTI), and their common inflammatory complications (IC), such as acute otitis media (AOM) and rhinosinusitis (RS), are the most common causes of consultation with primary care paediatricians. These infectious processes generally increase when children start schooling. During a recent systematic review, it was calculated that nursery attendance could be responsible for up to 50% of episodes of recurring infections in children,1 and increases the overall use of antibiotics up to three times more than in children not at school.2
Preventative measures against recurring colds and their complications are the topic of controversy. General hygiene procedures, and the use of nutritional supplements such as Vitamin A, Vitamin C, iron and minerals have a limited effect.3 Other measures include the use of preventative antibiotic, gamma globulin, immunostimulants, auto-vaccines, influenza vaccines and anti-pneumonia vaccines, all of which have a limited preventative effect. Surgical adenoid ablation has not been seen to reduce the number of URTI cases, despite initial expectations.4
In previous papers completed by this group on overweight and obese children and child asthma,5,6 it was observed that in addition to appropriate control of excess weight and bronchial hyper-reactivity, these patients showed a better response to recurring infections in childhood. These results, together with a number of reference documents regarding the anti-inflammatory properties of the Mediterranean diet,7,8 lead to the hypothesis that recurring colds and their subsequent complications may be exacerbated by a reduction in healthier, traditional eating habits, resulting in an abnormal and insufficient immune response and an imbalance in the inflammatory regulation system that protects the human body.9,10
The objective of this study was to assess the effects of dietary intervention, implemented via the educational programme “Learning to eat the Mediterranean Way”, in children with a high propensity to recurring colds and frequent inflammatory complications such as acute otitis media and rhinosinusitis.
Patients and methodsStudy designThe study was approved by the Ethics and Research Committees at Ciudad Real General University Hospital. The design consisted of a prospective, quasi-experimental analytical study or before-after comparison, completed in a primary attention paediatrics office. The sample included patients attending consultation between May 2009 and February 2015 with a particular propensity to recurring colds and frequent inflammatory complications. Consecutively all patients aged between 1 and 5 years who in the year prior to the study had presented at least three inflammatory complications in the last six months, or more than three in the last year, regardless of the number of URTIs presented. All the patients had been under the care of our primary care paediatricians for at least one year prior to the study.
Informed consent forms were completed by the parents and guardians. The study focused on dietary re-training based on the Traditional Mediterranean Diet (TMD) through the use of the nutritional education programme “Learning to eat the Mediterranean Way”.5,6 Patients were monitored over the course of one year, assessing growth using the ponderal index, clinical evolution, treatment requirements, adherence to the TMD and the level of satisfaction in their families, using the variables specified below.
This programme is based on a series of joint consultations with the paediatrician and the nutritionist, to which the whole family was invited. Consultations were held monthly for the first four months, and then bi-monthly until the end of the year. The first consultation consisted of an assessment of each child's eating habits and that of their families using frequency questionnaires and interviews, and changes in nutrition were advised based on the issues observed, using charts, recipes, sample menus, etc. Anthropometric measurements were taken and basic health matters explained such as the importance of breakfast, variety of menu, the balance between food consumption and energy expenditure, the quality of fats, proteins and carbohydrates, how to interpret labels, making shopping lists, etc. Subsequent consultations consisted of reiteration of nutritional advice and joint assessments of evolution by the pedestrian and the nutritionist.
This diet is based on the set of guidelines proposed by the Mediterranean Diet Foundation11,12 on its website, which has been declared Intangible Cultural Heritage by Unesco13 (Table 1). It is characterised by a diet rich in fresh natural foods such as fruit, vegetables, wholegrain cereals, pulses, extra virgin olive oil and nuts, low or moderate consumption of animal-sourced food such as fermented dairy products, fish, eggs and lean meat, and limited consumption of sugars, refined flours and pre-prepared food.
Mediterranean diet. 10 basic recommendations.
| 1. Use olive oil as your main source of added fat. |
| 2. Eat plenty of fruits and vegetables; fruits, vegetables, legumes and nuts. |
| 3. Bread and other grain products (pasta, rice, and whole grains) should be a part of your everyday diet. |
| 4. Foods that have undergone minimal processing, that are fresh and locally produced are best. |
| 5. Consume dairy products on a daily basis, mainly yoghourt and cheese. |
| 6. Red meat should be consumed in moderation and if possible as a part of stews and other recipes. |
| 7. Consume fish abundantly and eggs in moderation. |
| 8. Fresh fruit should be your everyday dessert and, sweets, cakes and dairy desserts should be consumed only on occasion. |
| 9. Water is the beverage par excellence in the Mediterranean Diet. |
| 10. Be physically active every day, since it is just as important as eating well. |
The principal variable is inflammatory complications of the upper respiratory tract, occurring after URTIs, principally in children taking the form of acute otitis media and rhinosinusitis.14–16
An episode of URTI was defined as two or more of the following: fever above 38°C, measured with a digital or tympanic thermometer, nasal congestion or breathing through the mouth, nasal secretion, odynophagia and coughing.17 The criteria for AOM were based on the guidelines of the American Academy of Paediatrics: 1. Acute onset; 2. Middle ear effusion indicated by bulging of the tympanic membrane, pathological pneumatoscopic findings, or otorrhoea; 3. Signs and symptoms of inflammation such as otolagia or evident erythema of the tympanic membrane. “Probable” cases of AOM were not taken into consideration, and AOM was considered “confirmed” if in addition to any of the above criteria URTI was present. RS was defined as per the Protocols of the Spanish Association of Paediatrics, as persistent daytime cough or rhinorrhoea for more than ten days, with no apparent improvement, in the context of an upper respiratory tract infection.18–21 The before and after diagnosis of AOM was established with the use of high resolution visual examination equipment, and tympanometry and audiometry apparatus (Macroview®, Micro-Tymp-3®, Audioscope®, all made by Welch\Allyn®).
The following secondary variables were considered: number of URTIs, emergency medical attention, number of prescribed symptomatic treatments (analgesics and antipyretics, mucolytics, antihistamines, antitussives, etc.), number of prescribed antibiotics. These factors were assessed per patient and per year. Finally, a subjective clinical assessment was completed by the sole paediatrician, including valuation of the effect of complicated episodes of colds (mild-1, moderate-2 or intense-3). In all cases, and for the duration of the study, the same paediatrician was responsible for the diagnosis, treatment and assessment of clinical evolution.
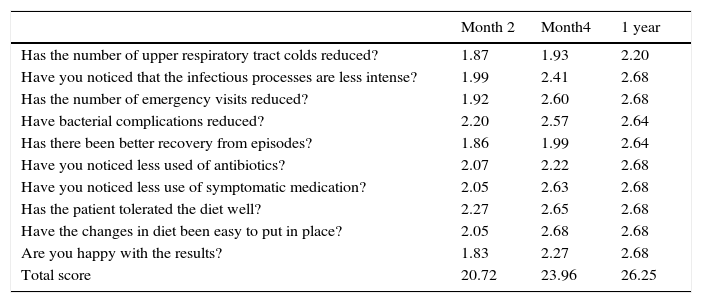
Family questionnaireIn order to assess patient clinical evolution and the level of adherence to the nutritional programme, we designed a questionnaire for parents/guardians to explore the symptoms relating to URTIs and IC. This is a subjective assessment of the clinical response in the children after exposure to common triggers of infection, and how after re-adjustment of the inflammatory system via the proposed eating habits, a more effective balance could be predicted. Each question was answered in line with the improvement observed: 3 – notable; 2 – reasonable; 1 – some; 0 – none (Table 2). Ten questions were posed regarding the past four weeks, with a possible score of between 0 (poor control) and 30 (good control). The sum of these ten scores was calculated, and the patient was considered to be well controlled with a total score above 20.
Evolution of family assessment questionnaire*
| Month 2 | Month4 | 1 year | |
|---|---|---|---|
| Has the number of upper respiratory tract colds reduced? | 1.87 | 1.93 | 2.20 |
| Have you noticed that the infectious processes are less intense? | 1.99 | 2.41 | 2.68 |
| Has the number of emergency visits reduced? | 1.92 | 2.60 | 2.68 |
| Have bacterial complications reduced? | 2.20 | 2.57 | 2.64 |
| Has there been better recovery from episodes? | 1.86 | 1.99 | 2.64 |
| Have you noticed less used of antibiotics? | 2.07 | 2.22 | 2.68 |
| Have you noticed less use of symptomatic medication? | 2.05 | 2.63 | 2.68 |
| Has the patient tolerated the diet well? | 2.27 | 2.65 | 2.68 |
| Have the changes in diet been easy to put in place? | 2.05 | 2.68 | 2.68 |
| Are you happy with the results? | 1.83 | 2.27 | 2.68 |
| Total score | 20.72 | 23.96 | 26.25 |
We collected body measurements, including weight, height, skin folds, arm, abdomen and waist measurements and used them to calculate body mass index (BMI), lean mass and body fat under standard procedure in use by our group.5,6
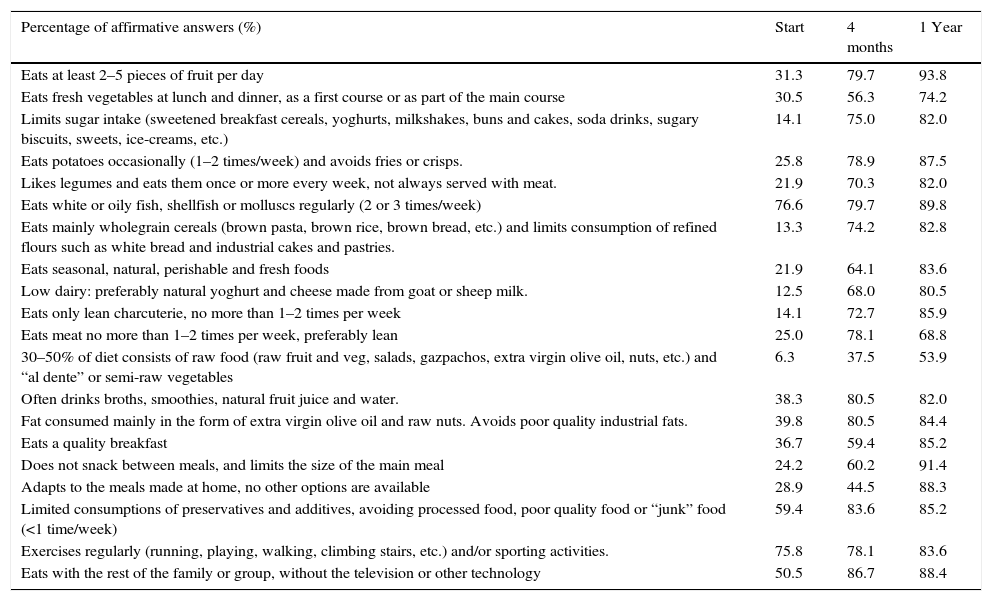
TMD adherence parametersAll participants received exactly the same nutritional information, targeted at the entire family, the main points of which are summarised in the Guidelines for the Mediterranean Diet.11 For the assessment of the new dietary habits of patients and their families, the KIDMED test was used. This test assesses the level of adherence to the Mediterranean Diet in children and teenagers.22–24 We also used a TMD test designed within our group, consisting of 20 questions, which we consider to be more TMD-specific (Table 3).
Traditional Mediterranean Diet Test (TDM).
| Percentage of affirmative answers (%) | Start | 4 months | 1 Year |
|---|---|---|---|
| Eats at least 2–5 pieces of fruit per day | 31.3 | 79.7 | 93.8 |
| Eats fresh vegetables at lunch and dinner, as a first course or as part of the main course | 30.5 | 56.3 | 74.2 |
| Limits sugar intake (sweetened breakfast cereals, yoghurts, milkshakes, buns and cakes, soda drinks, sugary biscuits, sweets, ice-creams, etc.) | 14.1 | 75.0 | 82.0 |
| Eats potatoes occasionally (1–2 times/week) and avoids fries or crisps. | 25.8 | 78.9 | 87.5 |
| Likes legumes and eats them once or more every week, not always served with meat. | 21.9 | 70.3 | 82.0 |
| Eats white or oily fish, shellfish or molluscs regularly (2 or 3 times/week) | 76.6 | 79.7 | 89.8 |
| Eats mainly wholegrain cereals (brown pasta, brown rice, brown bread, etc.) and limits consumption of refined flours such as white bread and industrial cakes and pastries. | 13.3 | 74.2 | 82.8 |
| Eats seasonal, natural, perishable and fresh foods | 21.9 | 64.1 | 83.6 |
| Low dairy: preferably natural yoghurt and cheese made from goat or sheep milk. | 12.5 | 68.0 | 80.5 |
| Eats only lean charcuterie, no more than 1–2 times per week | 14.1 | 72.7 | 85.9 |
| Eats meat no more than 1–2 times per week, preferably lean | 25.0 | 78.1 | 68.8 |
| 30–50% of diet consists of raw food (raw fruit and veg, salads, gazpachos, extra virgin olive oil, nuts, etc.) and “al dente” or semi-raw vegetables | 6.3 | 37.5 | 53.9 |
| Often drinks broths, smoothies, natural fruit juice and water. | 38.3 | 80.5 | 82.0 |
| Fat consumed mainly in the form of extra virgin olive oil and raw nuts. Avoids poor quality industrial fats. | 39.8 | 80.5 | 84.4 |
| Eats a quality breakfast | 36.7 | 59.4 | 85.2 |
| Does not snack between meals, and limits the size of the main meal | 24.2 | 60.2 | 91.4 |
| Adapts to the meals made at home, no other options are available | 28.9 | 44.5 | 88.3 |
| Limited consumptions of preservatives and additives, avoiding processed food, poor quality food or “junk” food (<1 time/week) | 59.4 | 83.6 | 85.2 |
| Exercises regularly (running, playing, walking, climbing stairs, etc.) and/or sporting activities. | 75.8 | 78.1 | 83.6 |
| Eats with the rest of the family or group, without the television or other technology | 50.5 | 86.7 | 88.4 |
For the calculation of sample size, significance was 0.05 and power 85%, assuming a reduction in the average number of IC per patient per year of one unit, and a typical deviation of 3.5 units, adjusted for a loss rate of 25%, resulting in a sample size of 115 patients. For results analysis the statistics package SPSS 15.0 was used. A descriptive analysis was completed with core tendencies and dispersions statistics for quantitative variables, and absolute and relative frequencies for qualitative variables. A comparison of the results for the different variables before and after intervention was completed using the Student's t test for paired data where the variables followed normal distribution, or using the Wilcoxon test in the case of abnormal variables, after checking using the Shapiro–Wilk test.
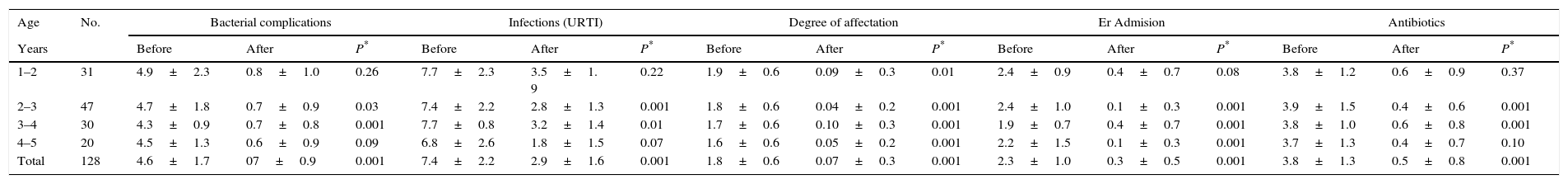
ResultsThe families of 145 patients meeting the inclusion criteria were invited to take part in the “Learning to eat the Mediterranean Way” programme, nine of whom declined. Of the 136 patients initially included, eight abandoned the programme, five due to social or personal difficulties with adhering to the diet and three due to disagreement with the limitation of certain foods. The study was therefore complete with a total of 128 patients, 65 of which were male and 63 female, with an average age of 2.9±1.24. Half of the patients were aged between 12 and 33 months at the start of the study. The results obtained were similar between the sexes, and therefore collated jointly. The anthropometric variables before the study, after four months and upon completion of the study are given in Table 4.
Anthropometric, clinical and therapeutic data (mean±SD).
| Age | No. | Bacterial complications | Infections (URTI) | Degree of affectation | Er Admision | Antibiotics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Years | Before | After | P* | Before | After | P* | Before | After | P* | Before | After | P* | Before | After | P* | |
| 1–2 | 31 | 4.9±2.3 | 0.8±1.0 | 0.26 | 7.7±2.3 | 3.5±1. 9 | 0.22 | 1.9±0.6 | 0.09±0.3 | 0.01 | 2.4±0.9 | 0.4±0.7 | 0.08 | 3.8±1.2 | 0.6±0.9 | 0.37 |
| 2–3 | 47 | 4.7±1.8 | 0.7±0.9 | 0.03 | 7.4±2.2 | 2.8±1.3 | 0.001 | 1.8±0.6 | 0.04±0.2 | 0.001 | 2.4±1.0 | 0.1±0.3 | 0.001 | 3.9±1.5 | 0.4±0.6 | 0.001 |
| 3–4 | 30 | 4.3±0.9 | 0.7±0.8 | 0.001 | 7.7±0.8 | 3.2±1.4 | 0.01 | 1.7±0.6 | 0.10±0.3 | 0.001 | 1.9±0.7 | 0.4±0.7 | 0.001 | 3.8±1.0 | 0.6±0.8 | 0.001 |
| 4–5 | 20 | 4.5±1.3 | 0.6±0.9 | 0.09 | 6.8±2.6 | 1.8±1.5 | 0.07 | 1.6±0.6 | 0.05±0.2 | 0.001 | 2.2±1.5 | 0.1±0.3 | 0.001 | 3.7±1.3 | 0.4±0.7 | 0.10 |
| Total | 128 | 4.6±1.7 | 07±0.9 | 0.001 | 7.4±2.2 | 2.9±1.6 | 0.001 | 1.8±0.6 | 0.07±0.3 | 0.001 | 2.3±1.0 | 0.3±0.5 | 0.001 | 3.8±1.3 | 0.5±0.8 | 0.001 |
| Age | No. | Weight (kg) | Height (cm) | IMC | Fat mass (%) | Lean mass (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| years | Begin | End | P* | Begin | End | P* | Begin | End | P* | Begin | End | P* | Begin | End | P* | |
| 1–2 | 31 | 10.8±1.8 | 13.6±2.2 | 0.36 | 0.79±0.1 | 0.91±0.1 | 0.10 | 16.8±1.6 | 16.2±1.5 | 0.82 | 15.1±2.9 | 15.6±3.7 | 0.77 | 9.1±1.3 | 11.4±1.8 | 0.27 |
| 2–3 | 47 | 12.5±1.8 | 14.9±2.1 | 0.18 | 0.88±0.1 | 0.97±0.1 | 0.93 | 15.9±1.4 | 15.6±1.2 | 0.35 | 14.6±3.2 | 14.4±2.5 | 0.024 | 10.7±1.5 | 12.8±1.7 | 0.34 |
| 3–4 | 30 | 16.2±4.6 | 18.7±5.1 | 0.64 | 0.99±0.1 | 1.08±0.1 | 0.73 | 16.2±2.7 | 15.8±2.6 | 0.80 | 15.1±4.3 | 14.5±4.5 | 0.93 | 13.7±2.9 | 15.8±3.2 | 0.70 |
| 4–5 | 20 | 21.3±4.3 | 24.5±5.2 | 0.29 | 1.13±0.1 | 1.21±0.1 | 0.69 | 16.4±1.7 | 16.4±1.8 | 0.95 | 16.7±4.1 | 16.7±3.7 | 0.34 | 17.7±3.4 | 20.4±4.1 | 0.39 |
| Total | 128 | 14.3±4.7 | 17.0±5.3 | 0.52 | 0.93±0.1 | 1.01±0.1 | 0.38 | 16.3±1.8 | 15.3±2.5 | 0.68 | 15.2±3.6 | 15.1±3.8 | 0.30 | 12.1±3.6 | 14.3±3.9 | 0.53 |
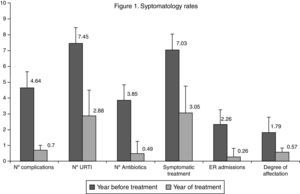
The average number of IC episodes after intervention reduced from 4.64±0.70 to 0.70±0.90, an average difference of −3.94±0.84 (IC95%: −3.74 to 4.08, p<0.001). Thus at the end of the study, 69 patients (54% IC95%: 45–63%) did not present any IC. Thirty-two presented one episode (25% IC95%: 17–33%) and 21 patients presented two episodes (16% IC95%: 10–23%). With regard to the number of URTIs, the average dropped from 7.45±1.74 to 2.88±1.60 over the year, an average difference of −4.57±0.14 (IC95%: −4.54 to 4.59, p<0.001). The differences in the values observed before and after intervention compared to the level of affectation, number of emergency visits, prescription of antibiotics and symptomatic treatment are shown in Fig. 1. All the variables showed a favourable evolution with significant differences (p<0.001). The results of the assessment questionnaires completed by the families are shown in Table 2.
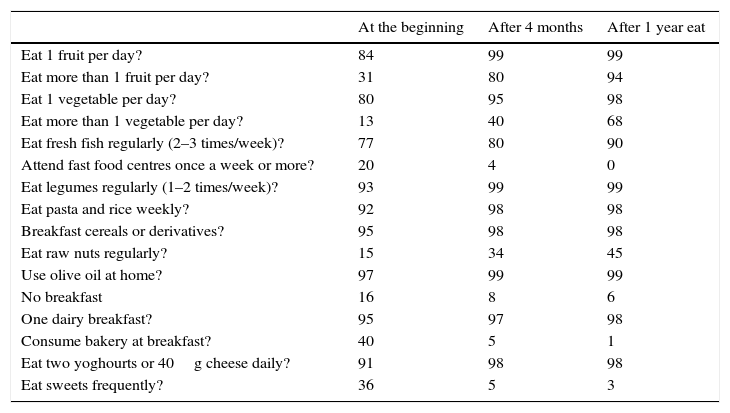
The anthropometric data evolved satisfactorily, in particular the increase in weight and height, the reduction in body fat and the increase in lean mass. Patient dietary habits assessed using the KIDMED index (Table 5) evolved positively from an average-high score (7.5±1.23, p<0.001) at the start of the programme to a good-optimum score (10.5±1.51, p<0.001) at the end of the study period. The TMD test also showed positive evolution, increasing from an average score of 6.7 points (poor quality diet), to 16.6, considered to be a good-optimum Traditional Mediterranean Diet (Table 2).
KIDMED test (percentage).
| At the beginning | After 4 months | After 1 year eat | |
|---|---|---|---|
| Eat 1 fruit per day? | 84 | 99 | 99 |
| Eat more than 1 fruit per day? | 31 | 80 | 94 |
| Eat 1 vegetable per day? | 80 | 95 | 98 |
| Eat more than 1 vegetable per day? | 13 | 40 | 68 |
| Eat fresh fish regularly (2–3 times/week)? | 77 | 80 | 90 |
| Attend fast food centres once a week or more? | 20 | 4 | 0 |
| Eat legumes regularly (1–2 times/week)? | 93 | 99 | 99 |
| Eat pasta and rice weekly? | 92 | 98 | 98 |
| Breakfast cereals or derivatives? | 95 | 98 | 98 |
| Eat raw nuts regularly? | 15 | 34 | 45 |
| Use olive oil at home? | 97 | 99 | 99 |
| No breakfast | 16 | 8 | 6 |
| One dairy breakfast? | 95 | 97 | 98 |
| Consume bakery at breakfast? | 40 | 5 | 1 |
| Eat two yoghourts or 40g cheese daily? | 91 | 98 | 98 |
| Eat sweets frequently? | 36 | 5 | 3 |
The core of our intervention was the nutritional education programme, which reinforced family awareness and skills, monitoring the adoption of a high quality, traditional, family-focused Mediterranean diet, without the need for counting calories or nutrients, and highly intuitive. The proposed diet was well tolerated, easily adaptable and did not pose major culinary difficulties. There was a satisfactory pondered growth rate, with patients gaining weight and height as expected. There was a significant increase in lean mass, matching our studies on excess weight and asthma in children.5,6 The notable increase in weight and lean mass correlated to the low incidence of infectious diseases and an increase in appetite compared to the previous year.
At the end of the study, episodes of URTIs had reduced in all patients, especially inflammatory complications. We opted for the use of the term “inflammatory complications” instead of “infectious complications” as the hypothesis with which we were working was based on the premise that inflammation (denoted by the suffix “-itis”: otitis, sinusitis, etc.) is caused by an alternation in the mechanisms involved in regulation of this phenomenon, while infectious agents are merely triggering factors in the process.
Particularly worthy of note is the reduction in clinical symptoms displayed in further episodes suffered by patients, together with fewer emergency visits and a drop in the use of antibiotics and symptomatic drugs. During the early months of nutritional therapy notable improvements could already be seen in comparison with the previous year. This resulted in a considerable increase in commitment to the diet, making it easier to monitor. At the start of the study, some patients had not yet been born. As intervention criteria were met, these patients were included in the study over a period of almost six years. Over the same period of time some children were included due to an increase in IC. Fifty percent of the children included in the study were aged between 12 and 33 months and such a satisfactory spontaneous evolution could not be expected. The other half of the sample was closer to reaching the age of natural immunity, but included children who were more severely and frequently affected than the average population. There was no difference between these age groups and all patients evolved satisfactorily. In terms of the season in which patients were included in the study, there was no significance, as all the children were studied before and after a full year-long period.
The notable reduction in the use of antibiotics (87.3%) and symptomatic drugs (56.6%) is of particular importance. More than 66.4% of patients did not require any antibiotic treatment over the course of the year.
The lack of a control group for this study makes it impossible to conclude that our intervention is the only determining factor in the response of the variables studied. However, the clinical history of the patients included in the study (those most severely and frequently affected), could not have led us to expect such a rapid and favourable evolution in symptoms, and the therapeutic effect of the diet therefore appears to be significant. This data suggests that the consumption of healthy food and/or the avoidance of other non-traditional foods may play an important role in the control of immune mechanisms and the regulation of inflammation. There is limited available literature on this topic.25–27 Our data cannot be compared with other studies, as we have not found clinical trials or prospective studies connecting adherence to a traditional diet with a reduction in inflammatory complications such as AOM and RS. The anti-inflammatory effects of the TMD are a controversial topic in the scientific community; PREDIMED, a key study carried out in adult patients identified the important of diet in the prevention of cardiovascular disease.7,28,29 Recently there have been meta-analyses, articles and prospective studies such as the one completed by our group,7 where the anti-inflammatory effects and notable reduction in cases of asthma in patients following the Mediterranean Diet have been identified.30,31 In the current feeding regime for babies aged over one year, breast milk is replaced with powdered cow's milk formula and with the “aid of cooking and blending” the variety of foodstuff that can be consumed has increased, introducing almost the same diet as an adult by the age of one. The industrial food sector has developed dietary patterns unknown until now to human infants, such as the fact that many children now almost exclusively eat industrially-prepared food, such as powdered milk, milk cereal preparations reconstituted with water, blended fruit, jars of baby food with all types of meat and fish, industrial dairy products, a multitude of industrial biscuits and cakes, charcuterie and endless industrial foods officially accepted for infant consumption.
Current eating habits have deeply altered the fundamentals of the traditional diet. The total antigenic load and the calorific density and nutrients have changed at molecular level. The new pattern encouraged by industrial development can be characterised by an increase in refined carbohydrates, a lack of natural fibre and fermented products, the use of more animal fats than vegetable fats, an increase or excessive use of animal protein, the use of a large number of food additives and an almost complete disappearance of fresh, seasonal food.32 From a purely nutritional perspective, the Traditional Mediterranean Diet uses food that is appropriately matured to be consumed directly, and the guarantee of having been the basis for human diet since pre-historic times.
A healthy diet can strengthen and balance the body's natural defences, providing all the nutrients required for proper self-healing. The TMD also provides important amounts of vitamins, minerals and anti-oxidants, many of which are indispensable co-factors in the enzymatic chemical reactions involved in inflammatory and defence processes.33
IgA is a highly specialised immunoglobulin in breastfeeding infants, as it is not transferred via the placenta, but may be found in the mother's breast milk. Production commences slowly and progressively after birth, meaning that at 12 months a child only has 20% of the IgA levels found in adults. Standard levels are not reached until the age of 10–12. These low levels of IgA may be related to the reduced need to recognise dietary antigens, due to historical feeding of children with a low antigenic load. Other immunoglobulins such as IgG and IgM are also found in lower levels at the age of 12 months, at 60–80% of those found in adults.34,35 All therapeutic initiatives against infections have focused on fighting external enemies – infection – with anti-microbial agents. In many infant patients there is a disproportionate inflammatory response to viruses, while in other patients there is scarcely any response at all. Is it possible that there is a hyper inflammatory response to small immune stimuli, due to alterations in the inflammation mechanisms? In other words, is it possible that a minor infection is not the cause of the illness, but the precipitating factor in hyper inflammatory response?36
Natural foods, such as symbiotic products, are beneficial for intestinal flora, adapted to the human race since historic times. This is very different to industrial microbiotics promoted by modern diets which are primarily putrefactive, due to their excess content in animal protein. Intestinal microbiotics depends on the food that we eat, and this plays a key role in the regulation of the immune system.37–39 The lack of antigen recognition by the Immune System of the Mucous Membranes, in particular secreted IgA, could be due to a hyper-inflammatory response to what should be minor antigenic stimuli.40
It is noteworthy that the patients in our study showed a high level of compliance in the Kidmed test at the start of the study, prior to initiating the dietary changes. In our opinion the Kidmed test,23 which measures the quality of the Mediterranean Diet, is not sensitive to all the nutritional factors proposed under the Mediterranean Diet Foundation Guidelines,11 as it does not take into consideration the percentage of fresh or processed foods, whether the cereals are wholegrain or refined, the type of dairy products consumed or the proportion of meat and charcuterie. For this reason, we created the TMD test to cover these areas; compliance evolved favourably until reaching the optimal Traditional Mediterranean Diet.
The limitations of this study include, in addition to the absence of randomised control group, the lack of systematic analyses for the assessment of immunity, which would have given more weight to the results. With this type of study it is not possible to control some confusing factors properly, such as, in this case, the natural reduction in the number of infections, or other factors such as greater care on the part of the parents. Nor was it possible to verify the consistency of diagnoses as would have been possible if more paediatric departments were available in order to control variability between observers.
We would like to highlight the great importance of our nutritional programme “Learning to eat the Mediterranean Way” in the modification an implementation of changes in lifestyle within families. We have worked hard with parents to change their eating habits and to increase their awareness of the benefits of the TMD. Compliance allowed us to achieve highly satisfactory results. The development of inflammatory diseases appears to have been facilitated by poor eating habits, not in terms of quantity, but in terms of the quality of the diet, resulting from the gradual abandoning of the TMD.
It can be concluded that the application of the Traditional Mediterranean Diet is highly effective in the prevention and treatment of patients suffering recurring colds and frequency inflammatory complications.
Conflict of interestThe authors have no conflict of interest to declare.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.