The mite alimentary canal contains plenty of microbiota. It is accepted that some of the microbial products function as adjuvants to speed up immune responses.
ObjectivesWe identified five bacterial proteins from dust mite, and Enterobacterial fimbriae H (FimH) was one of them. This study aims to test a hypothesis that the FimH protein enforces immunotherapy in asthmatic mice.
MethodsAsthmatic mice were treated by allergen specific immunotherapy (ASIT) with rDer f1/f2 or rDer f1/f2 plus FimH. Changes in inflammatory cell infiltration, airway hyperreactivity, frequency of Tregs, splenic CD4+IFN-γ+ cells, and serum levels of TGF-β, IL-10, IL-13 and IL-17A of asthmatic mice were checked.
ResultsASIT with rDer f1/f2 plus FimH reduced inflammatory cell infiltration, airway hyperreactivity (AHR), and levels of IgE and IgG1 compared to ASIT with rDer f1/f2 alone, but the levels of IgG2a increased. Asthmatic mice that underwent ASIT with rDer f1/f2 plus FimH showed increased frequency of Tregs, splenic CD4+IFN-γ+ cells, serum levels of TGF-β and IL-10; and deceased splenic CD4+IL-4+ cells, and serum levels of IL-13 and IL-17A. In vitro study showed FimH triggered IL-10 expression in a concentration dependent manner and facilitated the differentiation of Tregs.
ConclusionUsed as an adjuvant, FimH enforces the effect of ASIT in asthmatic mice via augmenting Tregs.
Allergic asthma is an IgE-mediated chronic inflammatory disease with the characteristics of pulmonary eosinophilic granulocyte infiltration and airway hyperreactivity.1,2 Its prevalence has increased rapidly in recent decades and is still on the rise worldwide. According to statistics, the treatment cost of allergic asthma reaches a billion euros in the European Union every year.3 Allergic asthma is the most common allergic disease in China4; the number of patients has reached about 30 million and children involved in asthma reached at least 10 million.5,6 Thus, asthma represents a major socio-economic and health burden worldwide.
Allergen specific immunotherapy (ASIT) is where the patient is treated with regular small amounts of the allergen of interest with the intention of achieving a tolerogenic response.7 Currently, the two administration techniques for allergic asthma involve subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT) but the techniques are developing with new approaches involving application of extracts to the skin with patches, injection into inguinal lymph nodes, and alterations to the allergen molecules by chemical treatment or recombinant technology.8 As the only effective approach to cure allergic asthma; ASIT is recommended by the World Health Organization (WHO) as a specific therapy for asthma.9–11 ASIT for asthmatic patients induced by dust mite is administered by vaccination using extracts from whole house dust mite (HDM) or using purified recombinant allergenic proteins. Whole HDM extracts contain the major HDM allergens, such as Dermatophagoides farinae (Der f) 1 and Der f2 and numerous irrelevant proteins. The latter may induce new allergic responses, which is a drawback of ASIT with HDM extracts as vaccines.12 Recombinant allergenic protein vaccines have defined antigenic components which are easily standardized, and cause fewer side effects during vaccination.13,14 However, it was reported that the therapeutic efficacy of an allergenic extract vaccine was better than recombinant allergenic protein vaccines,15 and the reason behind this phenomenon is unclear; it cannot be explained by the differences between dosage and the types of allergens.
Our previous studies primarily confirmed the abundant presence of microbiota in the guts of HDM; with the Enterobacter species the predominant genus.16 Enterobacterial fimbriae H (FimH) is the adhesion portion of type 1 fimbriae produced by most Enterobacteriaceae, which is a ligand of Toll like receptor 4 (TLR4).17,18 Current evidence suggests that TLR ligands are important immune adjuvants for vaccine development.19,20 TLR ligands activate the host immune system through TLRs present in various antigen-presenting cells such as monocytes, macrophages, and dendritic cells. As such, one TLR4 agonist adjuvant, monophosphoryl lipid A, has been approved by the Food and Drug Administration.21 Adjuvants can be used in ASTI to induce a quicker, more potent, and longer-lasting immune response.7 In this way, the use of a TLR4 ligand to enhance vaccine-specific responses is likely to beneficial for ASIT.
Therefore, we hypothesize that the products of microbiota in dust mites, in particular FimH, play an immune adjuvant role in ASIT. To test this hypothesis, we examined the ability of FimH to enforce the therapeutic effect of the dust mite vaccine-based ASIT in an asthmatic mouse model.
Materials and methodsPreparation of Der fDer f was from our own storage and maintained at 25°C and 75% relative humidity in incubators (LHL-113, ESPEC, Japan). Recombinant fusion proteins assembling Der f 1 and Der f 2 allergens (rDer f1/f2) were synthesized by Sangon Biotech Inc. (Shanghai, China) using a molecular cloning approach as previously described.22
Proteomic analysis of dust mite extractsProteomic analysis was performed to achieve more detailed information on the dust mite allergens. Briefly, whole dust mite extracts were separated by 2-dimensional polyacrylamide gel electrophoresis (2-D PAGE), all spots were collected for further analysis. After tryptic digestion, the peptides were analyzed by Matrix-Assisted Laser Desorption Ionization tandem Time-of-flight mass spectrometry (MALDI-TOF-MS) to identify bacterial proteins from dust mites.
Expression, purification and characterization of FimHTotal RNA was extracted from HDM using Trizol reagents (Invitrogen, USA). The first strand of cDNA was synthesized from total RNA using the RevertAid First-strand cDNA synthesis Kit (MBI Fermentas, USA). The cDNA was used as a template to amplify the gene sequence of FimH from the coding region by PCR with the following primers: forward: 5′-CCGAAGTGCATTACGACCTG-3′ and reverse: 5′-GATTTTCATCGCACCGTCCA-3′. The primers contained KpnI and XhoI restriction enzyme sites. The PCR consisted of 30 cycles of 1min at 94°C, 1min at 55°C, 1min at 72°C. After digesting with KpnI and XhoI (MBI, Canada), the fragments were cloned into the expression vector pET32a to construct the pET32a-FimH recombinant plasmid. Then pET32a-FimH was transformed into E. coli TG1 competent cells. A positive colony was identified by polymerase chain reaction (PCR) and double digestion. The recombinant plasmid of pET32a-FimH was transformed into Escherichia coli Rosetta (DE3) competent cells, and then incubated at 37°C in liquid LB culture medium overnight. Expression of the protein was induced with 1mmol/L IPTG (isopropylthio-β-d-galactoside). The protein was purified with a Chelating SFF (Ni) Column (Amersham, UK) according to the manufacturer's instructions. The purified protein was checked by 12% SDS-PAGE and quantified by the Bradford method. The endotoxin level of the purified recombinant protein was checked using the Limulus amebocyte lysate method.23 FimH was synthesized by Sangon Biotech Inc., using a molecular cloning approach.
Localization of FimH in the HDM alimentary canalThe recombinant FimH was injected into a New Zealand rabbit to obtain an anti-FimH polyclonal antibody. The titer of the antibody was measured by indirect enzyme-linked immunosorbent assay (ELISA). The antibody was labeled with fluorescein isothiocyanate (FITC). HDM was fixed in a cold formalin solution and embedded in paraffin and cut into 5μm sections. Then the sections were stained with the anti-FimH antibody labeled with FITC and examined for the localization of FimH in the HDM alimentary canal with fluorescence microscopy (Olympus, USA).
Immunization protocolsFemale BALB/c mice (Body weight was 16–22g, 6–8 weeks old) were purchased from the Animal Center of Guangdong Province and maintained in a pathogen-free environment. All experiments were approved by the Animal Ethics Committee at Shenzhen University. The experiments were performed in accordance with the Institutional Guidelines for the Care and Use of Laboratory Animals. Mice were randomly divided into four groups (n=6). Mice were anesthetized by inhalation of ether, and forceps were placed under the tongue of the anesthetized mouse to keep its mouth open; the antigen solution containing antigen plus the adjuvant (antigen:adjuvant=1:1, Freunds Complete Adjuvant, Beyotime Biotechnology, Shanghai, China), was administered by micropipette and maintained under the tongue for 1–2min in all mice except those in the healthy control group. The total volume of the antigen plus the adjuvant was kept to 7μL to avoid swallowing effects. The healthy control group was treated with phosphate buffered solution (PBS). The other three groups were sensitized intraperitoneally with 50μg HDM extract absorbed to 2mg alum on days 0, 7 and 14. From day 28 on (every two days, three administrations in total), mice were sublingually treated with PBS (asthma model group), 0.1g rDer f1/f2 (rDer f1/f2/ASIT group), or 0.1g rDer f1/f2 plus 0.03g FimH (rDer f1/f2+FimH/ASIT group), respectively. Seven days after the final immunization, mice were intranasally challenged with 50μg HDM extract daily for one week. Twenty-four hours after the last challenge, the airway hyperreactivity (AHR) was assayed. Twenty-four hours later, mice were sacrificed, and the lungs and spleens were removed for use. The spleen lymphocytes (106 cells/well) were prepared and cultured in RPMI-1640 supplemented with 10% fetal calf serum (FCS) in the presence of 10mg/mL Der f or PBS in a 96-well plate at 37°C for 72h. The CD4+ IL-4+ and CD4+ IFN-γ cells were measured by flow cytometer. Rat IgG2a was used to set the flow cytometer.
AHR assayAHR was measured using unrestrained whole-body plethysmography with a two-chamber system (Buxco, USA). Twenty-four hours after the last challenge, mice were put into the chamber and their breathing was monitored for 10min. When acclimated, the baseline responses were measured for 5min. Then the mice were subjected to 0.1ml of aerosolized PBS, followed by progressively increasing doses of methacholine (0, 6.25, 12.5, 25, 50 and 100mg/ml PBS). Responses were recorded for 5min for each case, with a short interval in between to allow return to baseline enhanced expiratory pause.
ELISABlood was collected from the mice by removing the eyeballs before sacrificing on day 48. After the blood was left at room temperature for one hour, the serum was separated at 400g and centrifuged at 4°C for 5min. 96-well plates were coated with 5μg/ml Der for serial dilutions of murine antibodies overnight at 4°C. Sera were diluted (1:100 for IgG1, IgG2a or 1:10 for IgE) in TBST/0.1% BSA and incubated overnight at 4°C. The plates were washed with PBST and incubated with horseradish peroxidase-conjugated anti-mouse IgG1, Ig2a or IgE (Southern Biotech, USA) for one hour at 37°C. The reaction was developed by adding TMB and stopped with 2M H2SO4. Then, the OD value was recorded with an ELISA Reader (Thermo) at 450nm.
Spleen lymphocytes were prepared as presented in the immunization protocols section above and the culture supernatants were collected and centrifuged at 400g at 4°C. The culture supernatants were lyophilized and stored at −20°C until they were used for cytokine assay. The levels of IL-13, IL-17A, IL-10 and TGF-beta in the culture supernatants were evaluated by sandwich ELISA (R&D) according to the manufacturer's instructions.
Lung histological stainingThe lungs were immediately removed after death and fixed in 4% cold formalin solution for 24h. The tissues were subsequently embedded in paraffin and cut into 5μm sections. Some of the sections were stained with hematoxylin-eosin to examine the histological changes using light microscopy. Then evaluation of the inflammation index was carried out using color segmentation (ImagePro plus 4.0, Media Cybernetics, L.P.). Other sections were stained with Periodic Acid Schiff (PAS) to identify the mucus-producing goblet cells in the airway mucosa. The PAS-positive cell count was based on counting nucleus to the nearest apical surface immediately beneath the PAS stain, whereas the ciliated cells were determined by counting nucleus nearest to the apical surface of the cells with cilia but without PAS staining. Both measurements were normalized to the length of the epithelium in millimeters. To ensure that we accurately quantify the amount of PAS staining, we also measured the area of the PAS stain and expressed this value as a percentage of the total epithelial cross sectional area using color segmentation (ImageProPlus 4.0, Media Cybernetics, L.P.).
Flow cytometrySpleen cells (2×106/sample) were permeabilized with fixation/permeabilization solution for 30min at 4°C, washed in perm/wash buffer, and then incubated with rat-anti-mouse CD4 mAbs-APC, rat-anti-mouse CD25 mAbs-FITC and rat-anti-mouse Foxp3 mAbs-PE for 60min at room temperature. Alternatively, Spleen cells (2×106) were incubated with rat-anti-mouse CD4 mAbs-APC and rat-anti-mouse IL-17A mAbs-PE-CY5 for 30min at 4°C. A control group was treated with rat IgG2a. Cells were fixed in formaldehyde and then analyzed on a BD-FACS Calibur (USA). Data were analyzed based on the percentages of Treg cells (CD4+CD25+Foxp3+T cells) and Th17 cells (CD4+IL-17A+T cells).
Bone marrow-derived dendritic cells (BMDC) and in vitro T-cell priming and polarizationBone marrow cells were generated from the femurs and iliac bones of female BALB/c mice and cultured in RPMI 1640 medium containing 10% FBS, 100U/ml penicillin/streptomycin, 20ng/ml recombinant mouse GM-CSF and 10ng/ml IL-4. One-half of the volume was replaced every other day. On day 8, the un-adhered cells were recovered for further study.
Splenocytes were harvested by mincing spleen tissue and passing the cells through a nylon mesh sieve. The cells were then cultured in RPMI 1640 medium containing 10% FBS. Prior to the assays, 24-well plates were coated with an anti-CD3 antibody (1μg/ml), then maintained at 4°C overnight and washed twice with PBS. Next, splenocytes (6×105) were co-cultured with BMDCs (2×104) in the presence of anti-CD28 (2μg/ml), TGF-β (20μg/ml), Der f 1 (20μg/ml) and/or FimH (10, 25 and 50μg/ml) for 5 days, then washed three times with PBS. Subsequently, the cells were collected and incubated with rat-anti-mouse CD4 mAbs-APC, rat-anti-mouse IL-10 mAbs-FITC and rat-anti-mouse IL-17 mAbs-PE to perform flow cytometry.
Quantitative real-time PCR (qRT-PCR)The expression levels of IL-10 and IL-27 were tested through qRT-PCR. Briefly, BMDCs were seeded into a six-well dish at a density of 5×105 cells per well and maintained at 37°C in 5% CO2. After overnight growth, the cells were stimulated with FimH (1, 5 and 10μg/ml) or LPS (1μg/ml). At four hours after stimulation, the cells were collected via centrifuge at 1500rpm. RNA extraction was performed according to the instructions of the RNA extraction kit (Fastagen, China, 220010), and the concentration was calculated at OD260. A total of 50μg of RNA was used for reverse transcription and RT-PCR (Transgen, China, AT341). GAPDH were used as an endogenous control for sample normalization, and the cDNA employed in these experiments was obtained from three independent replicates.
StatisticsThe data are represented as mean±SEM and analyzed by unpaired two-tailed t-test. p-Value less than 0.05 was considered significant. The data were analyzed by SPSS 17.0 statistical software. *p<0.05; **p<0.01; ***p<0.001; ns, no significant difference.
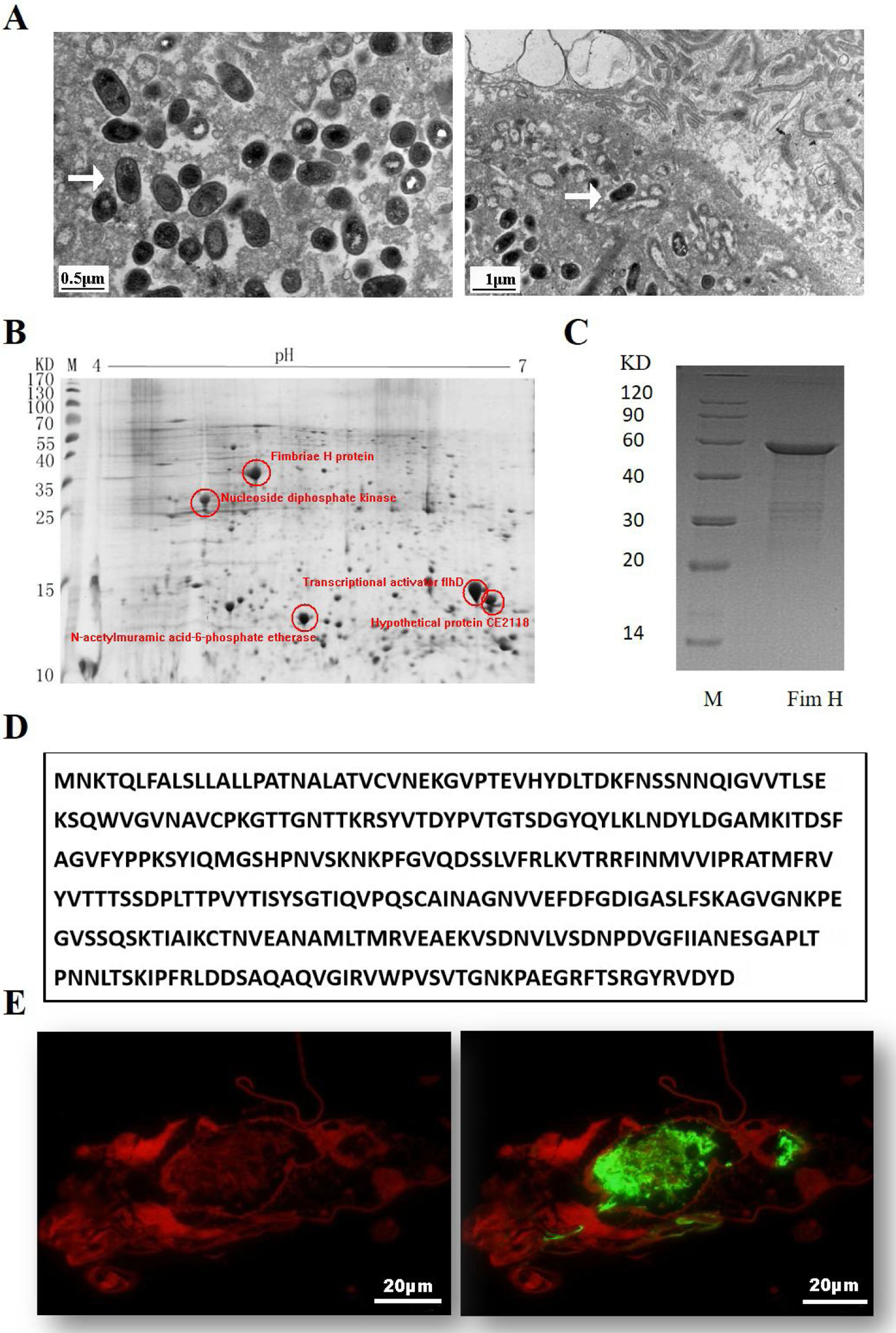
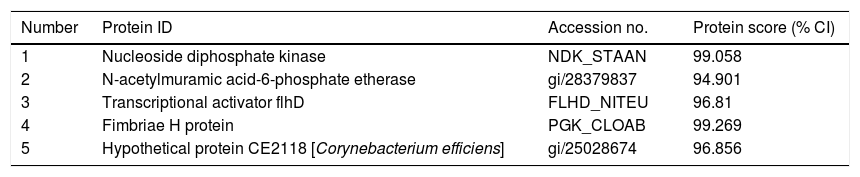
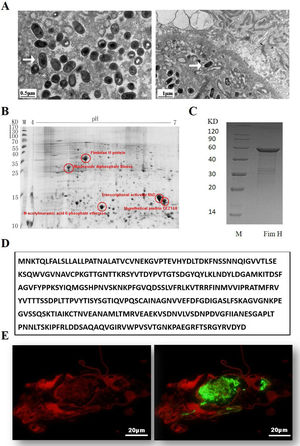
ResultsExpression of FimH and localization of FimH in the HDM alimentary canalTransmission electron microscopy (TEM) was employed to confirm whether bacteria existed in dust mites. As shown by Fig. 1A, there were many bacteria in the alimentary canal of dust mites. Subsequently, dust mite extracts underwent 2-D PAGE and Mass spectrometry, and five bacterial proteins were identified, FimH was one of them (Fig. 1B, Table 1). To confirm that FimH existed in the HDM alimentary canal, recombinant FimH and an anti-FimH antibody labeled with FITC were established in our laboratory (Fig. 1C). The recombinant FimH was expressed by E. coli Rosetta (DE3) and purified with Chelating SFF (Ni) Column. The molecular weight of FimH was about 53.3kDa (Fig. 1D). The purity of FimH exceeded 90%, and the endotoxin level of the purified recombinant protein was less than 0.1EU/ml. HDM sections stained with anti-FimH antibody labeled with FITC showed that FimH localized in the HDM alimentary canal (Fig. 1E).
Expression of FimH and localization of FimH in the HDM alimentary canal. (A) The results of TEM. (B) The graph of 2-D PAGE. (C) Recombinant amino acid sequence of pET32a-FimH. (D) The amino acid sequence of FimH. FimH was localized in the HDM alimentary canal, which was identified by using staining with anti-FimH antibody labeled with FITC. (E) The results of immunofluorescence the negative control was without FimH antibody.
Bacterial proteins from HDM identified by proteomics.
| Number | Protein ID | Accession no. | Protein score (% CI) |
|---|---|---|---|
| 1 | Nucleoside diphosphate kinase | NDK_STAAN | 99.058 |
| 2 | N-acetylmuramic acid-6-phosphate etherase | gi/28379837 | 94.901 |
| 3 | Transcriptional activator flhD | FLHD_NITEU | 96.81 |
| 4 | Fimbriae H protein | PGK_CLOAB | 99.269 |
| 5 | Hypothetical protein CE2118 [Corynebacterium efficiens] | gi/25028674 | 96.856 |
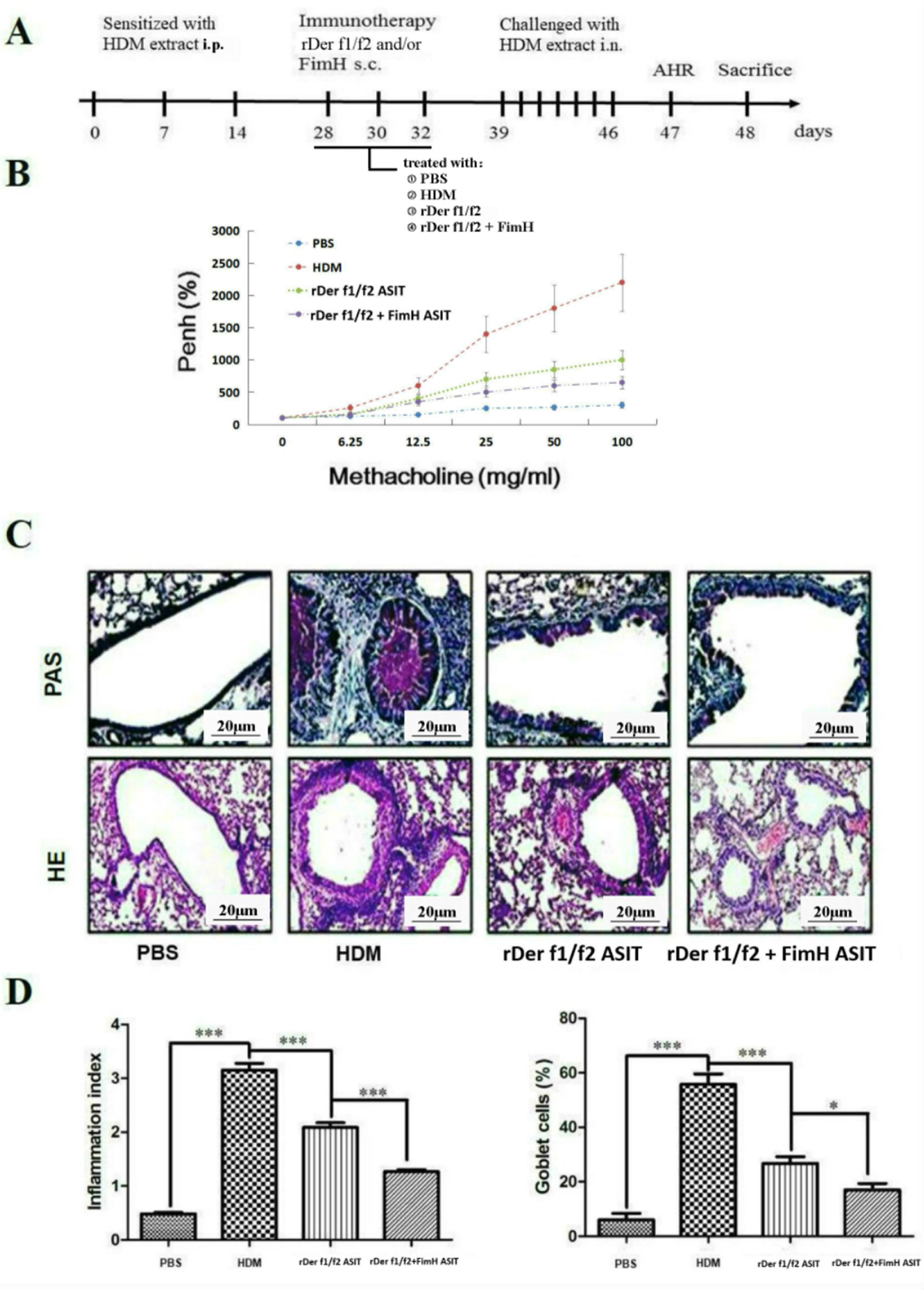
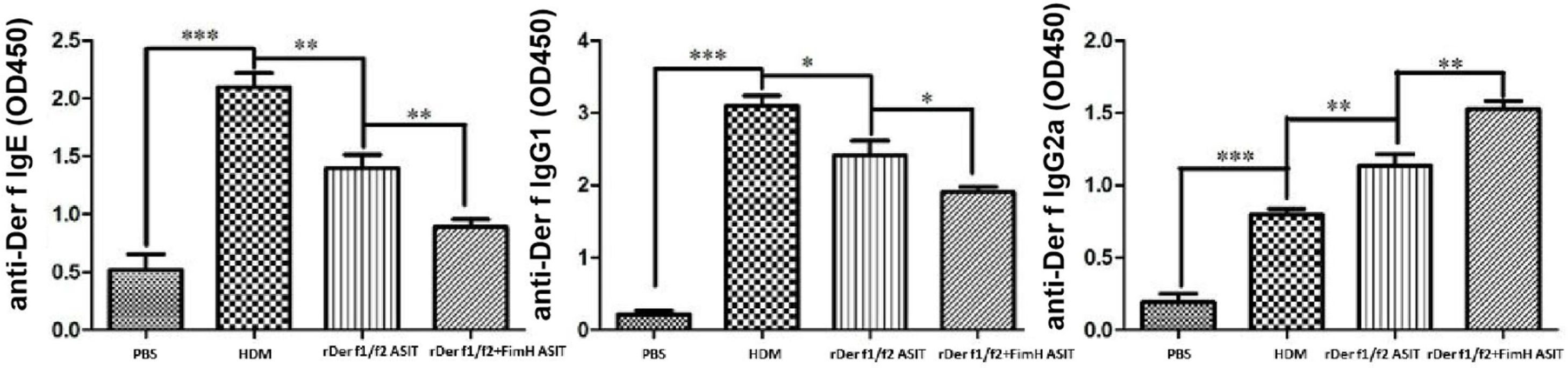
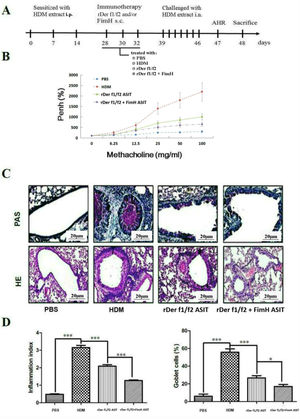
To assess the role of FimH in the therapeutic effect of the dust mite vaccine-based specific immunotherapy, HDM-sensitized mice were treated with rDer f1/f2, or rDer f1/f2 plus FimH, or PBS respectively (Fig. 2A). As shown by Fig. 2B, upon re-challenge with allergens, mice treated with rDer f1/f2 plus FimH showed a significant decrease in AHR when compared with the asthma model group and rDer f1/f2/ASIT group. Airway epithelial mucus hypersecretion and mucus plugging are prominent pathologic features of the asthmatic inflammatory airway disease. We observed that the mucus produced by goblet cells was large, oligomeric, O-linked glycoprotein with high molecular weight (2–40MDa) and size (0.5–10mm); which was confirmed by PAS staining (Fig. 2C). We found that the goblet cell count was decreased significantly in mice treated with rDer f1/f2 and rDer f1/f2 plus FimH as compared with the asthma model group (Fig. 2D). Lung sections stained with hematoxylin-eosin showed that ASIT with rDer f1/f2 or rDer f1/f2 plus FimH had a decreased lung inflammatory reaction (Fig. 2C, D). Serum antibody responses correlated with the Th2-biased cellular responses. Mice treated with rDer f1/f2 plus FimH showed lower levels of HDM-specific IgE, IgG1 vs. IgG2a when compared with the asthma model group and rDer f1/f2/ASIT group (Fig. 3).
FimH facilitates the therapeutic effect of dust mite vaccine-based specific immunotherapy. Mice were sensitized by intraperitoneal injection with HDM extract in Freunds Complete Adjuvant on days 0, 7, and 14. From days 28 through 32, mice were sublingually treated with PBS (asthma model group), or recombinant fusion proteins assembling Der f 1 and Der f 2 allergens (rDer f1/f2/ASIT group), rDer f1/f2 plus FimH (rDer f1/f2+FimH/ASIT group), respectively. From days 39 through 46, mice were intranasally challenged with HDM extract. (A) Animal model. On day 47, AHR was assayed. (B) AHR assay for mice. On day 48, mice were bled from the retro-orbital venous plexus and then sacrificed. (C) Lung sections were stained with PAS or hematoxylin-eosin (original magnification ×200). (D) The inflammation index was carried out using color segmentation according to hematoxylin-eosin staining, and percentage of the mucus-producing goblet cells was carried out using color segmentation according to PAS. All data which were processed by Graphpad software were mean±SEMs and generated from one of the three experiments (n=4–6). The significant differences between two groups were tested by unpaired two-tailed T-test. *p<0.05, ***p<0.001.
The antibody profiles in peripheral blood. Der f-specific antibodies (sIgG1, sIgE and sIgG2a) in sera were measured by ELISA. All data which were processed by Graphpad software were mean±SEMs and generated from one of the three experiments (n=4–6). The significant differences between two groups were tested by unpaired two-tailed T-test. *p<0.05, **p<0.01, ***p<0.001.
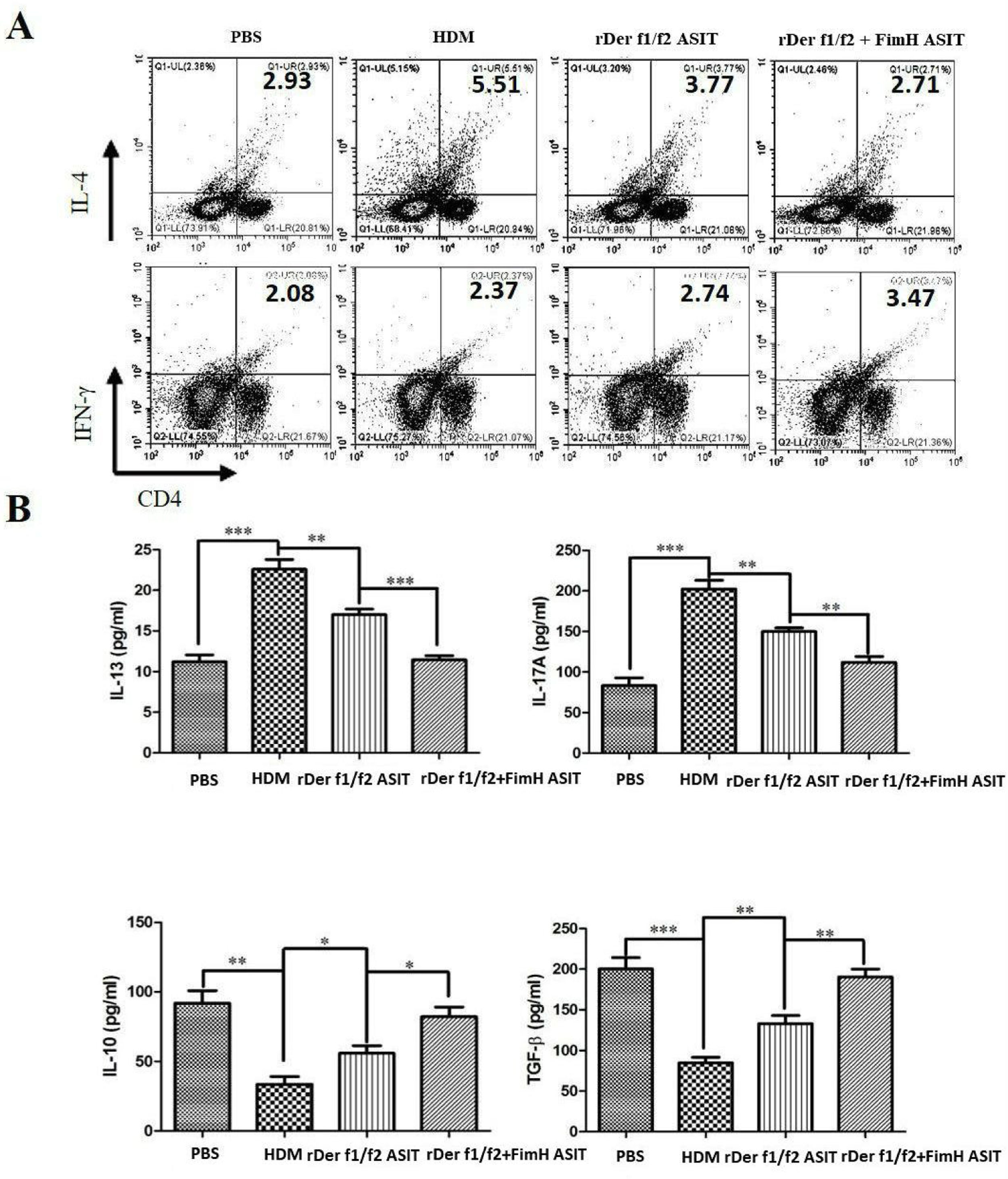
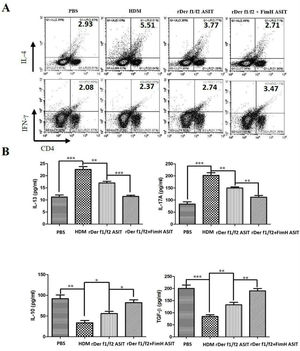
To evaluate the role of FimH in the regulation of inflammatory cytokines, CD4+IL-4+ and CD4+IFN-γ+ cells were measured by flow cytometry, and the levels of IL-13, IL-17A, TGF-β and IL-10 were detected by ELISA (Fig. 4). As shown by Fig. 4A, CD4+IL-4+ cells in rDer f1/f2 plus FimH group were less than in the asthma model group and rDer f1/f2/ASIT group, but CD4+IFN-γ+ cells in rDer f1/f2 plus FimH group were more than asthma model group and rDer f1/f2/ASIT group. Compared to the normal control mice, the levels of IL-13 and IL-17A in HDM-sensitized mice were increased significantly, but the levels of TGF-β and IL-10 in HDM-sensitized mice were decreased significantly (Fig. 4B). After ASIT with rDer f1/f2 or rDer f1/f2 plus FimH, the levels of IL-13 and IL-17A were decreased significantly versus the HDM-sensitized mice; the levels of TGF-β and IL-10 increased significantly versus those of HDM-sensitized mice (Fig. 4B). ASIT with rDer f1/f2 plus FimH had an effect on down-regulating the levels of IL-13 and IL-17A and up-regulating the levels of TGF-β and IL-10, which showed a better effect on mice treated with rDer f1/f2 alone (Fig. 4B).
FimH regulates the CD4+ T cell-related cytokines in mice with asthma. The splenic lymphocytes were cultured with HDM for 3 days, then analysis by flow cytometry. (A) CD4+IL-4+ and CD4+IFN-γ+ cells in spleen. (B) The levels of IL-13, IL-17A, TGF-β and IL-10 of normal mice or asthma mice accepted with ASIT with rDer f1/f2 or rDer f1/f2 plus FimH were measured by ELISA methods. All data which were processed by Graphpad software were mean±SEMs and generated from one of the three experiments (n=4–6). The significant differences between two groups were tested by unpaired two-tailed T-test. *p<0.05, **p<0.01, ***p<0.001.
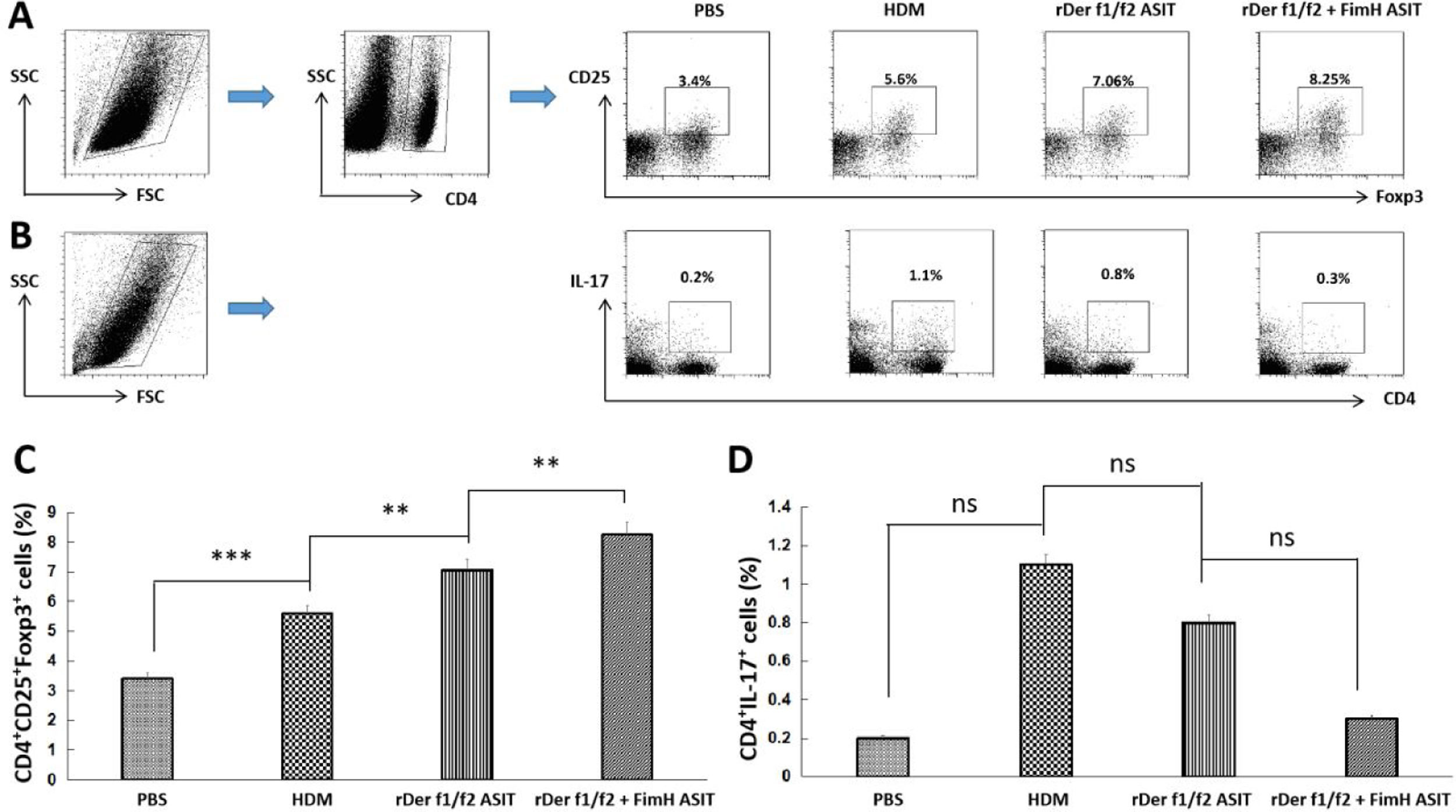
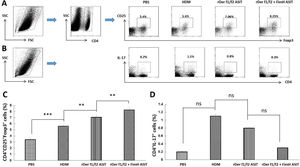
To estimate the potential regulatory function of FimH in Th17/Treg differentiation, counts of CD4+IL-17+ and CD4+CD25+FoxP3+ T cells in the spleen were performed by flow cytometry (Fig. 5A, C). CD4+IL-17+ cells in the control, HDM, rDer f1/f2 or rDer f1/f2 plus FimH groups were measured, and there were no significant differences among them (Fig. 5B, D). ASIT with rDer f1/f2 or rDer f1/f2 plus FimH markedly increased the frequency of CD4+CD25+FoxP3+ cells compared to the HDM-sensitized mice. Meanwhile, ASIT with rDer f1/f2 plus FimH had better effect on mice treated with rDer f1/f2 alone (Fig. 5A, C). It is indicated that FimH facilitates ASIT by increasing Tregs.
FimH augments Tregs in the spleen. The counts of CD4+IL-17+ T cells (Th17) and CD4+CD25+FoxP3+ T cells (Treg) in spleen of normal mice or asthma mice underwent ASIT with rDer f1/f2 or rDer f1/f2 plus FimH were measured by flow cytometry. (A) The representative flow profiles of Tregs. (B) The representative flow profiles of Th17. (C) Statistic results of Tregs. (D) Statistical results of Th17. All data which were processed by Graphpad software were mean±SEMs and generated from one of the three experiments (n=4–6). The significant differences between two groups were tested by unpaired two-tailed T-test. **p<0.01; ***p<0.001; ns, no significant difference.
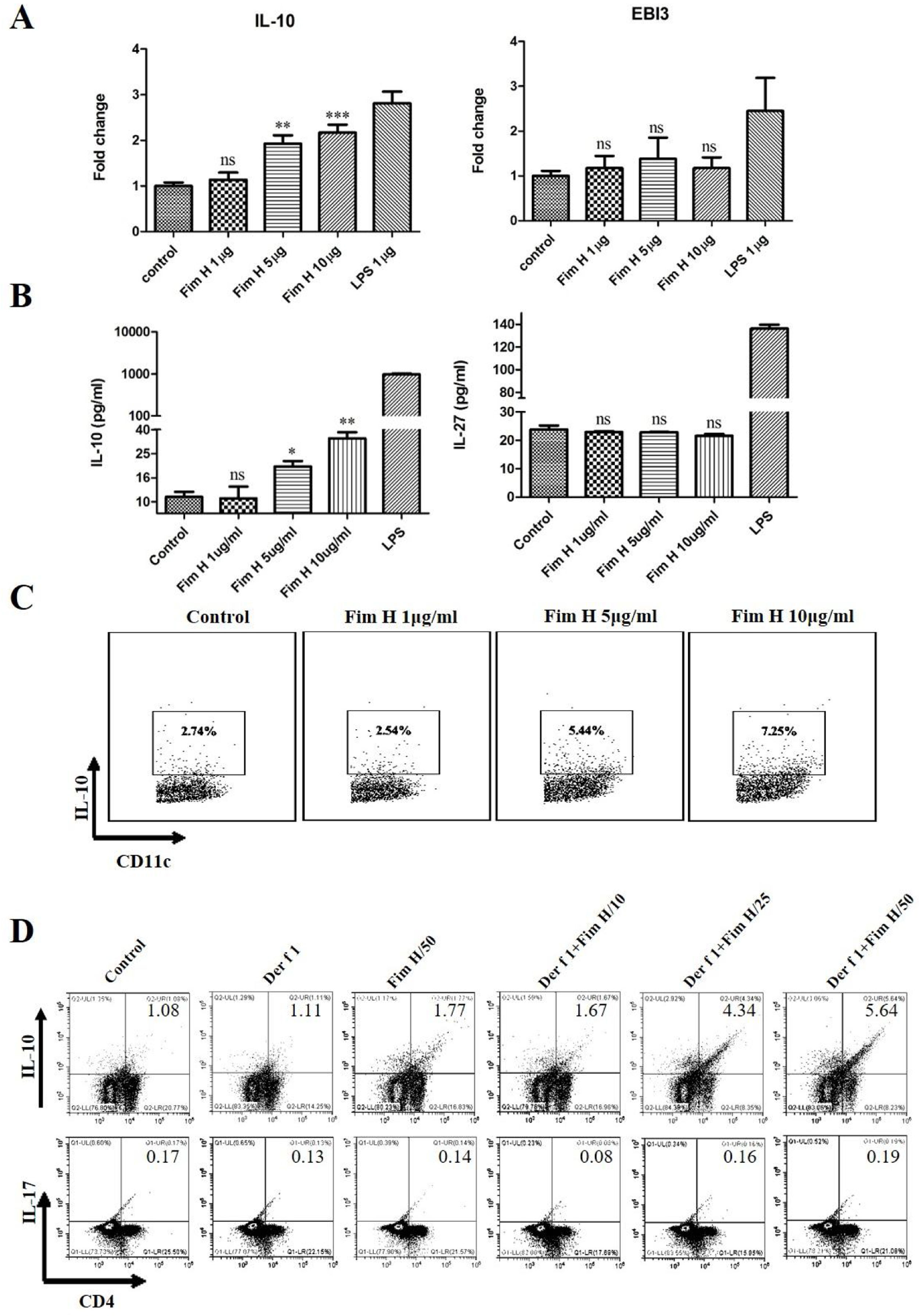
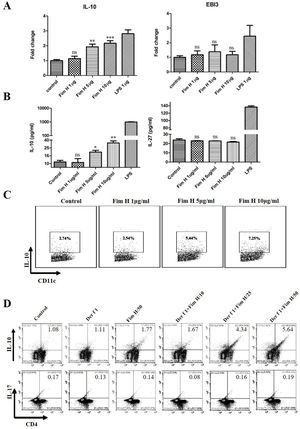
Based on the data above, we hypothesized that FimH could trigger inducible Tregs. Some researchers have clarified that the IL-10+ DCs play an important role in the development of Tregs, and IL-27 from DCs regulates the differentiation of Th17.24,25 Therefore, we tested the IL-10 and IL-27 levels in BMDCs treated with different concentrations of FimH by RT-PCR and ELISA. As shown in Fig. 6A, IL-10 mRNA was increased in a concentration dependent manner, but IL-27 mRNA was not different. The proteins levels of IL-10 and IL-27 were consistent with the results from qRT-PCR (Fig. 6B). Moreover, the data from the flow cytometer also confirmed that FimH triggered IL-10 of BMDCs (Fig. 6C). Subsequently, BMDCs and splenic lymphocytes from naïve mice were co-cultured in the presence of TGF-β, CD3 and CD28, then treated with Der f 1 and/or different concentration FimH. As shown in Fig. 6D, CD4+ IL-10+ cells were significantly increased by Der f 1 plus FimH treatment compared with Der f 1 alone and controls. However, CD4+ IL-17 cells were not different (Fig. 6D). Therefore, FimH may trigger IL-10 expression of DCs and inducible Tregs to facilitate ASIT.
FimH induces IL-10 from DCs and Tregs in vitro. BMDCs were treated with PBS, or different concentration FimH, or LPS for 4h, and then qRT-PCR performed. (A) The results of qRT-PCR. After 24h, the supernatants were collected to perform ELISA. (B) The results of ELISA. After 24h, the cells stained with CD11c and IL-10 to perform flow cytometer. (C) The results of flow cytometer. BMDCs and spleen lymphocytes were co-cultured in the exist of Der f 1 and/or FimH for 5 days, then the cells were collected to stain with anti-CD4, anti-IL-10 and anti-IL-17 to perform flow cytometer. (D) The representative flow profiles. The data (mean±SEMs) were generated form three independent experiments and processed by Graphpad software. The significant differences between two groups were tested by unpaired two-tailed T-test. *p<0.05; **p<0.01; ***p<0.001; ns, no significant difference.
In this study, we developed an HDM allergy model in order to evaluate the role of FimH as an adjuvant in vaccine-based ASIT. The results suggest that the therapeutic effect of ASIT with rDer f1/f2 plus FimH was much better than using rDer f1/f2 alone.
The results of this study suggest that ASIT with adjuvant FimH improved the response to the ASIT therapy in this model. This agrees with previous studies that investigated another TLR4 agonist, monophosphoryl lipid A, which showed that that adjuvant skewed the immune response toward Th1 and Treg pathways and decreased specific-IgE production, while improving bronchial hyper-reactivity and lung inflammation in mouse models.26,27 Monophosphoryl lipid A is the only currently approved TLR4 agonist for use an adjuvant.21 The mice treated with ASIT in this study showed decreased goblet cell count in the lungs compared to the model group and a decreased inflammatory reaction. While FimH adjuvant treatment showed lower levels of HDM-specific IgE, IgG1 vs. IgG2a when compared with the model group and the rDer f1/f2/ASIT group. This suggests that overall, ASIT improved the allergen-specific airway hyperreactivity, as was seen in the meta-analysis of clinical studies.28 This was then improved further by FimH.
Some debate remains over the mechanisms involved in ASIT; however, it is clear that the early tolerance phase involves Treg cells.7 Treg cells expressing Foxp3 or IL-10, or both molecules, are associated with the suppression of lung inflammation in humans by increasing Treg cell numbers in individuals in responding to ASIT.29,30 Therefore, Treg cells are important immune regulatory cells in ASIT.31 Active Treg cells can inhibit Th2 responses and the activation of mast cells, basophil granulocytes and eosinophil granulocytes directly.32,33 Meanwhile, Treg cells contribute to the immunoglobulin class switching in B cell from IgE to IgG4 and the restoration of peripheral immune tolerance.20 Our data show that ASIT with rDer f1/f2 plus FimH increased the numbers of Treg cells and was much better than the ASIT with rDer f1/f2 alone. The results suggest that FimH contributes to the induction of Treg cells. Our in vitro study also confirmed FimH could induce Tregs.
Th17 cells are a subset of CD4+ T cells characterized by the synthesis of IL-17A, IL-21, and IL-22, and the expression of IL-23R and CCR6 receptors.34 IL-17A is a pro-inflammatory cytokine that induces the synthesis of TNF-beta, IL-1α, IL-6, IL-8, and GM-CSF. Accumulating evidence suggests that activation of the IL-17-producing cells may be associated with the neutrophilic inflammatory responses and the development of severe forms of asthma.35,36 We found that Th17 cells treated by ASIT with rDer f1/f2 plus FimH and rDer f1/f2 alone had similar results.
There are abundant microbes in the guts of HDM,16 and plenty of microbial components may exist in the vaccines extracted from HDM. Our previous studies found that the Enterobacter species is the predominant genus of HDM gut microbes.16 Enterobacterial FimH is a ligand of TLR4; the signal of TLR4 can induce Th1 responses or Treg cells depending on the immune microenvironment, which plays critical roles in immune activation or immune tolerance.30,37,38 Some researchers suggest that the activated TLR4 signaling pathway can induce the differentiation of Treg and inhibit the differentiation of Th17 cells in autoimmune disease or respiratory diseases.38,39 Thus, we presume that the binding of FimH to TLR4 may enhance the therapeutic effect of dust mite vaccine-based ASIT on asthma through regulating the differentiation of Treg.
This study has some limitations. As an animal model-based study, the results may not accurately reflect the clinical situation due to lack of investigating SLIT or SCIT. The recombinant proteins used did not undergo detailed analysis to ensure the quality of the recombinant protein. Analysis of bronchoalveolar lavage fluid and the histological examination of lung sections were not undertaken in this study.
In conclusion, our data show that FimH can enforce ASIT via regulating the differentiation of Treg cells in asthmatic mice. We plan to use this data to assist in future studies designing vaccines to be used in ASIT.
FundingThis study was supported by grants from the China Postdoctoral Science Foundation (2016M592473), Natural Science Foundation of China (Nos. 91442118, 91542104 and 31400786), Guangdong Scientific Technology Social Development Project (Nos. 2013B3180002, 2013B031800023), Science and Technology Planning Project of Guangdong Province (No. 2014B090901041), Guangdong Foreign Scientific Technology Cooperative Project (No. 2013B051000088), Shenzhen Scientific Technology Basic Research Projects (Nos. JCYJ20120830091748817, JCYJ20160407130202989, JCYJ20140828163633992, JCYJ20140828163633991), Nanshan District Creative Mechanism Promoted Subsidized Project/Nanshan District Innovation and Development Subsidized Project (No. KC2014JSCX0055A), and State Key Laboratory of Respiratory Disease Open Fund (No. 2014SKLRD-005).
Author contributionsXiaomeng Yang and Hui Wang wrote the main manuscript and performed the experiments presented in Figs. 3–6. Dan Zhao, Xiaoyu Liu, Junyi Wang, Xiefang Yuan, Min Zhang prepared Figs. 1 and 2 and analyzed the data. Guoping Li, Zhigang Liu, Pingchang Yang and Pixin Ran designed the project, supervised the experiments and wrote the manuscript. All authors reviewed the manuscript.
Conflicts of InterestThe authors have no conflict of interest to declare.