INTRODUCTION
Asthma is the leading cause of chronic illness of childhood, affecting 7-12 % of children globally with an increasing morbidity and prevalence1,2. Although the genetic predispositions for atopy and bronchial hyperresponsiveness play a role in pathogenesis of asthma, some avoidable environmental factors provoke an inflammatory response and are known to exacerbate symptoms of asthma3-5. Substantial scientific evidence has proved passive smoking to be an important environmental risk factor in children spending 70-80 % of their time at home6-9. Although individual studies had conflicting results, a meta-analysis showed passive smoking not to be a causal factor for asthma but a risk factor increasing the severity, frequency of attacks and hospitalizations in already established asthmatics. However the question of whether an acute attack can be induced by acute smoke exposure remains unanswered10-12.
Documentation of smoke exposure requires objective markers. Cotinine, a metabolite of nicotine, is a widely used indicator to objectively quantify cigarette smoke exposure as it can be measured in body fluids accurately with a half-life of 10-37 hours in smokers and longer in non-smokers13-18.
Several studies have consistently showed that 70-75 % of children are exposed to tobacco smoke in Turkey7. The specific aims of our study were a) to investigate whether acute exposure of tobacco smoke can start an attack in asthmatic children chronically exposed to passive smoke, and b) whether questionnaires given to parents with high anxiety levels during the acute attack can reflect exposure objectively.
PATIENTS AND METHODS
The study population consisted of thirty-two asthmatic children who were consecutively admitted to the emergency clinic with an acute asthma attack between March 1996 and September 1996. Study was approved by the investigational committee of the hospital and written consents were obtained from the parents and the patients. Patients who were recruited into the study were diagnosed with asthma according to the guidelines of the international pediatric asthma consensus group having at least one previous episode of physician diagnosed acute asthma attack19.
A questionnaire including questions regarding smoking habits of all household members, housing conditions, and child's medical history was given to the parents on admission. Number of cigarettes smoked daily was coded into four intervals (1 to 10, 10 to 20, 20 to 30, and > 30). Educational status of the parents was described by calculating the total years of education. In terms of housing conditions, heating types and total number of rooms in the household were recorded.
Children were asked to provide a urine sample as soon as possible once they were admitted to the emergency department. Small amounts of urine such as two milliliters were required for analysis. The urine was refrigerated immediately, frozen within 12 hours, and kept frozen at 40 °C until analyzed. Urinary cotinine and creatinine levels were measured during the acute asthma attack and four weeks after the attack when the patients were free of asthma symptoms.
Urinary cotinine was measured in each sample by Double Antibody I-125 radio-immunoassay (DPC-LA, USA). The Double Antibody procedure is a liquid-phase radio-immunoassay, wherein I-125 labeled cotinine competes for a fixed time with cotinine in the patient urine sample for antibody sites. After incubation, the antibody-bound fraction is separated from the free fraction. Finally, the antibody-bound fraction is precipitated and counted. Patient sample concentrations are read from a calibration curve. The antiserum is specific for cotinine and other nicotine metabolites, with very low cross-reactivity to other compounds that might be present in patient samples. Urinary creatinine was measured with the use of a commercially available kit (Crescent Diagnostics, USA). Cotinine levels were then standardized by dividing the cotinine levels with creatinine. Results were expressed as cotinine/creatinine ratio (CCR). Limit of detection was determined to be 5 ng/mL of urine. All samples were analyzed without knowledge of the exposure status of the subjects. The level of 30 ng/mg was used to categorize subjects into exposed and unexposed, based on the report of Henderson et al20. Serum Ig E levels were measured during asymptomatic period by ELISA technique. Atopy status was defined by history, eosinophilia and high Ig E levels according to the patient's age.
STATISTICAL ANALYSIS
Statistical analysis was performed using SPSS for Windows statistical package. Continuous variables were compared by t-tests. Analysis of categorical variables was done by the chi-square. Spearman's rho and Mann-Whitney tests were used to correlate the parental report of exposure with urinary CCR results. Statistical significance was defined as p < 0.05.
RESULTS
Over a six-month period, 32 children with asthma were enrolled in the study. Of all the patients, 62.5 % were male. Mean age was 5.7 ± 3.2 years, ranging between 1 and 14 years. Mean attack rate was 3.5 ± 3.8 per year. Thirty-seven percent of the patients had not received any kind of anti-asthmatic treatment before admission to the emergency department. Thirty-one percent of children were given inhaled steroids which was the main drug used by this group of asthmatics.
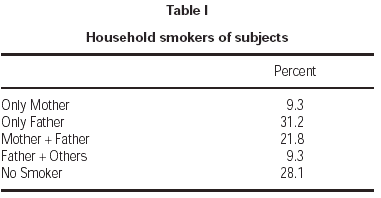
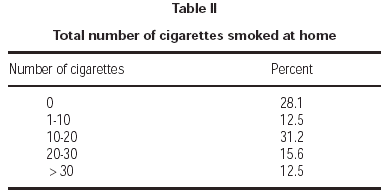
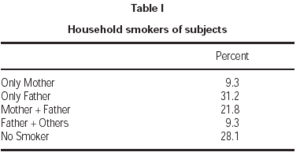
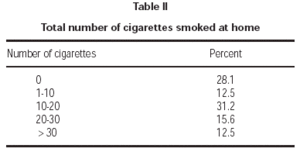
Seventy-one percent of children had at least one smoker parent at home. Fathers were the principal smoking parent at home, with a smoking rate of 62.3 %. Smoking rate among the mothers was 31.1 % (table I). Average number of cigarettes smoked daily by mothers and fathers were 2.1 ± 4.8 and 10.6 ± 12.2, respectively. Total number of cigarettes smoked at home was 14.4 ± 14.8 on average. Majority of patients (71.8 %) were exposed to less than 20 cigarettes daily (table II).
Table I Household smokers of subjects
Average period of education for mothers and fathers were 6.4 ± 3.1 and 8.7 ± 3.7 years, respectively. In terms of educational status there was a direct negative relationship for fathers between educational status and smoking habits, however this relation was inverse for mothers (p: 0.03, r: 0.39 and; p: 0.03, r: 0.36 respectively). In other words, the more educated mothers tended to smoke more. Prevalence of parental smoking was similar in treated and non-treated group, but the amount of cigarette exposure was significantly related to the previous treatment modalities. Children who had no anti-asthma treatment were exposed to cigarette smoke more than the children who had been given treatment. Average number of daily cigarettes smoked at home was 21.2 ± 15.6 for untreated group, but it was 11.7 ± 12.5 for the treated (p: 0.02, r: 0.42).
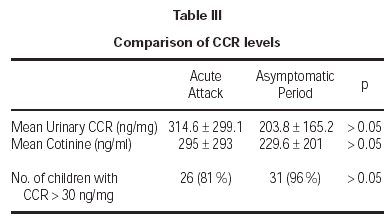
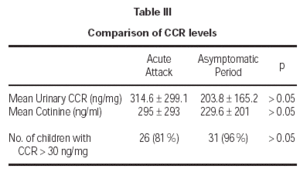
Cotinine levels were 295 ± 293 ng/ml and 229.6 ± 201 ng/ml during the acute attack and asymptomatic period, respectively (table III). These values were standardized according to urinary creatinine levels. All measurements for urinary creatinine were in normal range, between 0.5 mg/dL and 1.8 mg/dL. Mean urinary CCR levels were 314.6 ± 299 ng/mg during the acute attack and 203.8 ± 165.2 ng/mg during the asymptomatic period. Although cotinine and CCR levels during acute attack seemed to be higher, there was no significant difference between the objective measures of smoke exposure during acute attacks and asymptomatic periods (p > 0.05). Cut-off value for exposure was determined to be 30 ng/mg previously20. Exposure was shown for 81 % and 96 % of the children during acute attack and asymptomatic period, respectively (p > 0.05). There was no significant association between age and CCR levels (p > 0.05). The findings did not differ when data were analyzed after gender breakdown.
Sixty-seven percent of patients were atopic. There was no difference in CCR levels between atopic and non-atopic patients, during acute attacks and asymptomatic periods (326.4 vs. 311.6 and 202.3 vs 204.7, respectively).
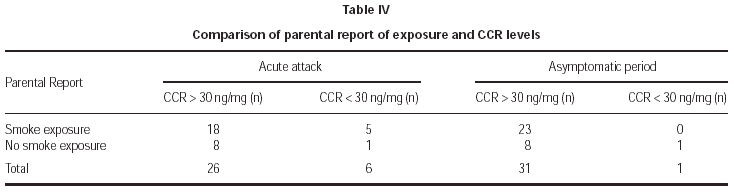
Parental report of smoke exposure showed no significant correlation with objective measurement of exposure both in acute attacks and asymptomatic periods (table IV). Nine parents reported no exposure during acute attacks and asymptomatic periods but only one patient was found as non-exposed in that group. There was no significant association between total number of daily smoked cigarettes at home obtained by questionnaire and CCR levels (p > 0.05).
In terms of housing conditions, there was no significant relation between CCR and number of rooms per person (p > 0.05). Wood-burning stove was the most common type of heat source (75 %) followed by central heating (25 %). There was no relation between the type of heating and CCR levels (p > 0.05).
DISCUSSION
Passive smoke exposure is a well-known risk factor for acute respiratory tract infections in children8,9. Although it was shown that passive smoke exposure aggravates symptoms in asthmatic children, effect of sudden heavy smoke exposure on precipitating acute asthma attack has not been documented21. In our study, we were also unable to show such an effect of sudden heavy smoke exposure. In other words, acute asthma attack did not coincide with sudden heavy smoke exposure in already smoke-exposed asthmatic children.
Questionnaire results showed that 71 % of all children had at least one smoker parent at home. This rate is similar to the other studies performed in Turkey7,22. It has been shown that children whose mothers are smokers had approximately 2.5 times greater risk to develop asthma than children whose mothers did not smoke1. Increasing prevalence of maternal smoking is a significant problem especially for child-bearing age group of women in many developing countries under the pressure of tobacco industry.
Previously diagnosed and treated asthmatic children are expected to have a lower prevalence of parental smoking due to the medical advice. Although parental education on the hazardous effects of smoking on their asthmatic child is one of the critical points that should be focused on in asthma management, it has been shown that asthma education programs might fail to involve parents who smoke23. In our study there was no difference between previously treated and non-treated groups in terms of passive smoke exposure supporting either inadequate education by the physicians or parental non-compliance. In previously treated group only the reported amount of cigarettes smoked at home was lower however the objective measurement of smoke exposure did not confirm the reported information. We speculate that despite the recommendations of the health staff, parents either only reduced the number of cigarettes they smoke or reported decreased amount of smoke exposure24.
Cotinine had been shown to be an objective indicator of passive smoke exposure15. CCR level is above 500 ng/mg among active smokers. A cut-off level of 30 ng/mg has been reported to distinguish children exposed to tobacco smoke from those unexposed by Henderson et al20. In contrast to this, some studies have shown that cotinine is measurable, sometimes at high levels, in children with no reported exposure at home25. In our study although CCR levels found in acute attacks were higher than the asymptomatic periods, difference was not statistically significant. In a study of 609 children admitted to emergency room, Reese et al. found elevated levels of CCR among children with bronchiolitis compared to children with non-respiratory symptoms26. Ehrlich et al investigated whether recent elevations of passive smoke exposure triggered attacks of asthma requiring visits to the emergency room. Although mean CCR level in symptomatic asthmatic children was 46 ng/mg, it was 38.5 and 25.8 ng/mg in asymptomatic asthmatic group and healthy subjects, respectively. These levels were not significantly different from each other. The only difference they found was the higher rate of smoker mothers among asthmatic children11. In a study by Ogborn et al. 56 asthmatic children were investigated during acute attack and well visits for a possible triggering factor of passive smoke exposure. They found a mean CCR level of 93 ng/mg during the acute attack and it was 97 ng/mg during the well visit12. Willers et al demonstrated a mean cotinine level of 10 ng/mL in the urine of asthmatics and this was significantly higher than normal children who had a mean cotinine level of 4.8 ng/ml (p < 0.005)27.
In our study, the CCR levels detected were generally higher than those reported in other studies. A study from Japan by Matsukura et al. showed that mean CCR level among passive smoke exposed non-smoker adults was 680 ng/mg28. That is the highest level reported in non-smokers. There is possibly a regional variation in passive smoke exposure all around the world. In our country also like in Japan, risk of passive smoke exposure is higher and this may lead to high levels of CCR in passive smokers. The oldest child with a CCR of > 500 ng/mg was a 4.5 years old boy and the highest level of CCR (1000 ng/mg) was detected in a one-and-a half-year-old child. Although passive smoke exposure at daycare could be responsible for such high levels of cotinine, none of these children had attendance of daycare. This finding demonstrates that such high levels can be found in passive smoke exposed children and may be the cut-off level for active and passive smoking should be revised. In homes where parents are heavy smokers, passive smoking level of CCR can overlap with previously defined active smoker levels.
We were unable to demonstrate any significant difference between CCR levels measured during acute attacks and asymptomatic periods. Ogborn et al. could not demonstrate such a difference either12. In this study, authors suggested that among asthmatic children with chronic exposure, an asthmatic attack might not be triggered by discrete increases in passive smoke exposure. Instead, they suggested that any further insult, such as cold, weather change, or exercise may simply induce the asthma attack, in the child who would not respond this way without chronic smoke exposure. Although this may be a possible explanation, we should also consider the possibility of parental avoidance of smoking at the onset of first symptoms of the attack. This kind of behavior may cause reduced levels of CCR during an attack and mask the real effect of passive smoke exposure. Such effect can only be demonstrated by a biological marker with a relatively long half-life. Hair cotinine levels may be used to assess cumulative exposure over months and can be a more accurate method for detecting long-term exposure29.
Reliability of parental report of exposure compared to cotinine levels has been studied and some studies have shown objective markers should be used for detecting the rate of exposure17. In our study, parental report of passive smoke exposure was 71 %, but as an objective method of assessing the degree of smoke exposure, CCR levels revealed that 81 % and 97 % of children were exposed to passive smoke during acute attacks and asymptomatic periods, respectively. Especially, the parental report of "no exposure" was found unreliable. Cunningham et al observed that parents reported misleading information about their smoking habits17. Previous studies revealed that reliability of parental report of passive smoke exposure was especially lower in asthmatic children. Clark et al. have found the exposure rate as 69 % by measuring the salivary cotinine whereas it was only 31 % in questionnaire forms30. However, Chilmonczyk et al. suggested that questionnaires are reliable for detecting the rate of smoking18. They showed that only 14 percent of children had higher levels of CCR in the non-exposed group.
Another important factor that may affect the passive smoke exposure is the number of rooms per person at home. We could not show any relation between the number of the rooms per person and CCR levels. This may be explained partly by the structure of the families in our country. Family relations are so close that although they live in separate houses, many relatives visit each other so frequently and thus a questionnaire form asking also the smoking habits of visiting persons is also needed.
In conclusion, this study revealed that the degree of passive smoke exposure was not higher during the acute attack in asthmatic children chronically exposed to passive smoke at home. This finding can also be explained by the parental avoidance of smoking at the onset of the first symptoms of the attack, which would prevent detection by a biological marker with a relatively short half-life. CCR levels detected in this study were generally higher than those reported in other studies. Since the smoking rate in the crowded urban environment of our subjects is high, this discordance may have been caused by differences in the general prevalence of smoking in our country. Approximately 75 % of children are exposed to cigarette smoke in Turkey, so that passive smoking is often almost unavoidable in public places. Questionnaires given to parents with high anxiety levels during the acute attack were found unreliable compared to CCR levels in our study. We speculate that a biological marker with a relatively longer half-life such as hair cotinine may be much more useful in such studies. Also, instead of a questionnaire form it is crucial to use an objective method of assessing the degree of smoke exposure especially in asthmatic groups.