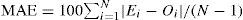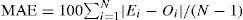Although the harmful impact of environmental tobacco smoke on respiratory health in early childhood is well known, its effect in adolescence is still ambiguous. This study aims to examine if parents’ and household tobacco smoking habits influence asthma, rhinitis and eczema in early adolescence in The Republic of Macedonia, as a country with a very high rate of household tobacco smoke exposure despite the smoking cessation campaign, and low prevalence rates of asthma, rhinitis and eczema.
MethodsChildren aged 13–14 years (n=3026) from randomly selected schools in Skopje, the capital of Macedonia, completed by themselves the standardised International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three written questionnaires on asthma, rhinitis, eczema and potential environmental risk factors. Maternal and paternal tobacco smoking habits and the number of smokers in households were separately correlated to current and ever-diagnosed asthma, rhinitis and eczema by odds ratios (OR, 95% CI) with and without adjustments for potential confounders using binary logistic regression.
ResultsThe maternal smoking habit was significantly positively associated only with current night dry cough apart from chest infection (aOR: 1.26; 1.03–1.54; p=0.026). No significant association was observed in relation to the other studied variables with either parental smoking habits or the number of smokers in the household.
ConclusionHousehold tobacco smoking habits were not found to have a significant influence on asthma, rhinitis and eczema in young adolescents. The established results point out the dominant influence of maternal smoking on cough as an unspecific asthma symptom.
Environmental tobacco smoke (ETS), as the most common indoor air pollutant, has been postulated as one of the risk factors for respiratory diseases, especially asthma.
Much of the current evidence from cross-sectional and longitudinal observation studies suggests an increased risk of new-onset asthma as a consequence of ETS exposure in pregnancy and early childhood and increased respiratory symptoms such as cough, wheeze, phlegm, respiratory infections of later ETS exposure, the effect of which appears to diminish with the increasing age of the child.1 However, there is still debate as to whether ETS exposure beyond infancy can initiate asthma or simply acts as a trigger of exacerbations in established asthma.1–3 In a recently published meta-analysis,4 it was concluded that ETS in developing asthma is not limited to younger children, certain high-risk populations, or prevalent cases. Moreover, a recently published review article5 pointed out the increased risk of asthma in adults exposed to ETS. Maternal smoking has been reported to have a stronger effect than other household members’ smoking in the induction of asthma in children.3
Given current evidence, parental smoking either before or after birth is unlikely to increase the risk of allergic sensitisation in children.5–7 There have been several studies with conflicting results performed on associations of ETS exposure with rhinitis and eczema symptoms. Some studies have reported a positive association of ETS with rhinitis symptoms8,9 or eczema symptoms and ever-diagnosed eczema,10,11 while other studies6,12 have reported no association between them.
Compared to asthma, rhinitis, and eczema prevalence rates worldwide,13–15 the Republic of Macedonia appears to have a moderately low prevalence of current asthma and low prevalence rates of current rhinoconjunctivitis and eczema symptoms.16 Despite the smoking cessation campaign worldwide, the prevalence of ETS at home in early adolescence in Macedonia has been demonstrated to be very high.17,18 On the other hand, dietary antioxidants intake has been documented to be high,18 which may be explained by the geographical area in which our country is situated and its climate.
This study aims to contribute to the inconsistent reports in the literature about the association of ETS exposure with asthma, rhinitis and eczema in children, especially at older ages, investigating 13/14 year-old children in Skopje, the Republic of Macedonia, where ETS exposure is still very high and the prevalence rates of asthma, rhinitis, and eczema are low.
Subjects and methodsThis study was conducted in Skopje, the capital of the Republic of Macedonia, as part of the Macedonian arm of the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three, and in accordance with its methodology.19,20
Self-reported data obtained through the standardised ISAAC Phase Three written questionnaires on asthma, rhinitis, eczema and environmental risk factors by 3026 children aged 13/14 years from 17 randomly selected schools was used in the analysis. According to ISAAC protocol, questionnaires were translated into Macedonian and back into English, and were used in original form without supplemental questions. Informed consent from parents was obtained prior to data collection, which was performed between December 2001 and March 2002. The response rate was 90.9%.
Outcome measures used in the study were the prevalence rates of current wheeze, current speech-limiting wheeze, current dry night cough, current nose and eye symptoms, current symptoms of eczema, and ever-diagnosed asthma, hay fever and eczema as defined previously.13–15,19 Severity indices related to rhinitis and eczema symptoms were subcategorised into ‘none’ and ‘any’. Due to the low prevalence rates, current severe nose symptoms were defined as interference of nose symptoms with daily activities in a moderate and a great amount while current severe eczema symptoms as current eczema symptoms associated with any sleep disturbance (less than one night per week, and one or more nights per week).
The maternal and paternal tobacco smoking habits as well as the number of smokers in household (none, one smoker, two and more smokers in household including the parents) were separately assessed in relation to asthma, rhinitis, and eczema prevalence rates. Out of the whole group of respondents, only 7.0% lived in a family with ≥3 smokers and therefore we subcategorised the smokers in the family by none, one, and ≥2.
Gender; fruit and vegetables and cereals intake per week in the previous 12 months; gas and wood used for cooking at home; wood/coal/oil used for heating at home; cat and dog ownership in the previous 12 months; maternal educational level; and existence of siblings, were all used as potential confounders. The current fruit and vegetables and cereals intake, as an indicator of external antioxidant intake, was classified as frequent (≥3 times a week) versus infrequent (never/occasionally and 1–2 times a week). Gas and wood used for cooking and wood/coal/oil used for heating at home were employed as inhaled oxidants, while current cat and dog ownership as sources of indoor allergen exposure. The mother’s educational level and existence of siblings, as familiar socioeconomic status indicators, were classified as tertiary (university) versus secondary/primary educational level and presence versus absence of siblings.
Of all respondents, 1568 (51.8%) were boys and 1458 (48.2%) were girls. Their mean age was 13.45 years (SD 0.50).
Ethical approvalThe conduction of the ISAAC Phase Three in The Republic of Macedonia has been approved by The Ethics Committee at the Medical Faculty and The Ministry of Education and Science, Skopje, The Republic of Macedonia.
Statistical analysesMissing or “any other” responses were part of the denominator for calculation of prevalence figures.21
A chi-square test was conducted to test for statistical significance in the comparison of the prevalence rates between the symptoms of the investigated diseases. The associations of maternal and paternal smoking habits, and the number of smokers in the household—both unadjusted and adjusted for the same confounders (gender, current fruit and vegetables and cereals intake, gas/wood cooking and heating exposure at home, current cat and dog ownership, maternal educational level, siblings)—with asthma and rhinitis and eczema were analysed by odds ratios with 95% confidence interval (OR, 95% CI) in binary logistic regression in SPSS 11.0 for Windows. No maternal or paternal smoking habits and no smokers in household were separately used as referent categories in statistical analyses. A resulting P-value of <0.05 was considered significant.
The number of respondents whose asthma, rhinitis and eczema were attributable to maternal smoking and ≥2 smokers in household was derived from the formula for attributable cases22,23:
where Ac is the number of attributable cases, Ne is the number of cases exposed to ETS and RR is relative risk. Relative risks were calculated using estimated odds ratios according to the formula22:where p is the outcome incidence in the unexposed referent group (i.e., cases who were not exposed to ETS).ResultsThe established prevalence of ever-diagnosed asthma (1.7%) was much lower than the prevalence rates of current wheeze (8.8%) and current night dry cough apart from chest infection (16.5%) (p=0.000 for both) and was more similar to the prevalence of current speech-limiting wheeze as a parameter of asthma severity (1.2%). The prevalence of night dry cough was also much higher compared to the prevalence of current wheeze (p=0.000). In contrast, the prevalence of ever-diagnosed hay fever (6.7%) was significantly higher compared to the prevalence of current nose and eye symptoms (5.8%) (p=0.000). Current severe nose symptoms were observed in 2.5% of the respondents. We documented prevalence rates of 2.7% and 1.0% for current symptoms of eczema and current severe eczema symptoms respectively, which were significantly lower compared to the prevalence of ever-diagnosed eczema (3.7%) (p=0.000 for both).
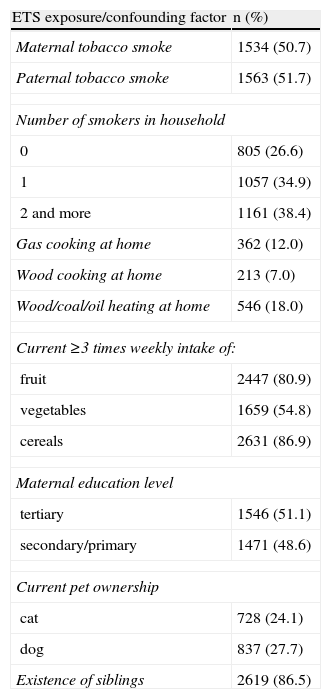
The majority of the respondents reported the presence of at least one smoker in the family (73.3%), frequent current fruit and cereals intake and the existence of siblings. One half of the respondents were exposed to maternal or paternal smoking and had a tertiary educated mother. Low indoor air pollution exposure by cooking and heating fuel, and pets exposure were documented (Table 1).
Prevalence rates of household smoking habits and confounding factors (written questionnaire) in 3026 children aged 13–14 yrs in Skopje, Republic of Macedonia, 2002
| ETS exposure/confounding factor | n (%) |
| Maternal tobacco smoke | 1534 (50.7) |
| Paternal tobacco smoke | 1563 (51.7) |
| Number of smokers in household | |
| 0 | 805 (26.6) |
| 1 | 1057 (34.9) |
| 2 and more | 1161 (38.4) |
| Gas cooking at home | 362 (12.0) |
| Wood cooking at home | 213 (7.0) |
| Wood/coal/oil heating at home | 546 (18.0) |
| Current ≥3 times weekly intake of: | |
| fruit | 2447 (80.9) |
| vegetables | 1659 (54.8) |
| cereals | 2631 (86.9) |
| Maternal education level | |
| tertiary | 1546 (51.1) |
| secondary/primary | 1471 (48.6) |
| Current pet ownership | |
| cat | 728 (24.1) |
| dog | 837 (27.7) |
| Existence of siblings | 2619 (86.5) |
ETS=environmental tobacco smoke; Current=in the last 12 months.
In girls compared to boys we observed almost equal prevalence rates of maternal (51.3% vs. 50.1%, respectively) and paternal (51.9% vs. 51.4%, respectively) smoking habits as well of number of smokers in household (35.3% vs. 34.6% for one smoker and 38.9% vs. 37.8% for ≥2 smokers in household, respectively).
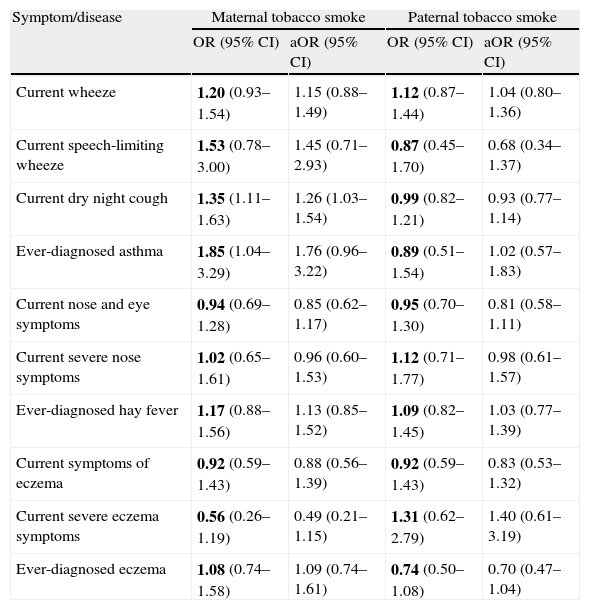
The relationship of maternal and paternal smoking habits with asthma, rhinitis, and eczema is shown in Table 2. A significant positive association was established between maternal smoking and current night dry cough apart from chest infection and significance decreased after controlling for potential confounding factors (OR: 1.35; 1.11–1.63; p=0.003 and aOR: 1.26; 1.03–1.54; p=0.026, respectively). Ever-diagnosed asthma was positively associated with maternal smoking, but the significance disappeared after adjustment (p=0.037 and p=0.066, respectively).
Relationship of parental smoking habits with asthma, rhinitis, and eczema in 3026 children aged 13–14 yrs in Skopje, Republic of Macedonia, 2002
| Symptom/disease | Maternal tobacco smoke | Paternal tobacco smoke | ||
| OR (95% CI) | aOR (95% CI) | OR (95% CI) | aOR (95% CI) | |
| Current wheeze | 1.20 (0.93–1.54) | 1.15 (0.88–1.49) | 1.12 (0.87–1.44) | 1.04 (0.80–1.36) |
| Current speech-limiting wheeze | 1.53 (0.78–3.00) | 1.45 (0.71–2.93) | 0.87 (0.45–1.70) | 0.68 (0.34–1.37) |
| Current dry night cough | 1.35 (1.11–1.63) | 1.26 (1.03–1.54) | 0.99 (0.82–1.21) | 0.93 (0.77–1.14) |
| Ever-diagnosed asthma | 1.85 (1.04–3.29) | 1.76 (0.96–3.22) | 0.89 (0.51–1.54) | 1.02 (0.57–1.83) |
| Current nose and eye symptoms | 0.94 (0.69–1.28) | 0.85 (0.62–1.17) | 0.95 (0.70–1.30) | 0.81 (0.58–1.11) |
| Current severe nose symptoms | 1.02 (0.65–1.61) | 0.96 (0.60–1.53) | 1.12 (0.71–1.77) | 0.98 (0.61–1.57) |
| Ever-diagnosed hay fever | 1.17 (0.88–1.56) | 1.13 (0.85–1.52) | 1.09 (0.82–1.45) | 1.03 (0.77–1.39) |
| Current symptoms of eczema | 0.92 (0.59–1.43) | 0.88 (0.56–1.39) | 0.92 (0.59–1.43) | 0.83 (0.53–1.32) |
| Current severe eczema symptoms | 0.56 (0.26–1.19) | 0.49 (0.21–1.15) | 1.31 (0.62–2.79) | 1.40 (0.61–3.19) |
| Ever-diagnosed eczema | 1.08 (0.74–1.58) | 1.09 (0.74–1.61) | 0.74 (0.50–1.08) | 0.70 (0.47–1.04) |
OR=Odds ratio; aOR=adjusted OR; CI=confidence interval; Current=in the last 12 months.
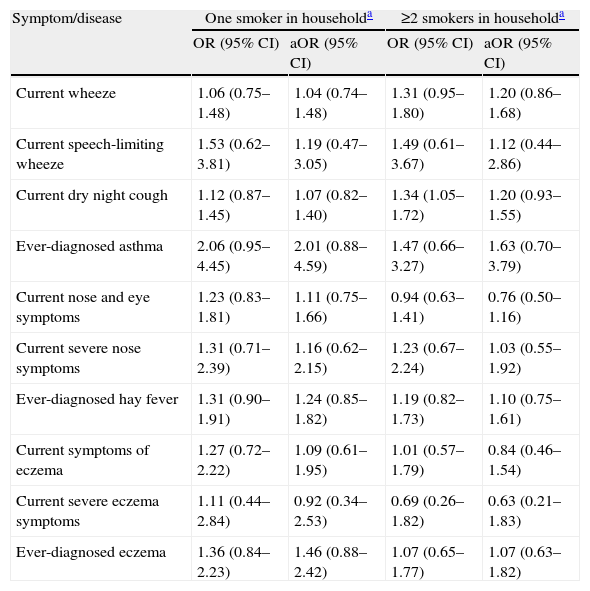
Two or more smokers in household significantly increased the risk of current night dry cough only before adjustment for confounders (p=0.019 vs. p=0.166). No other significant associations between number of smokers in household and asthma, rhinitis, and eczema before and after adjustment were found (Table 3).
Relationship of the number of smokers in household with asthma, rhinitis, and eczema in 3026 children aged 13–14 yrs in Skopje, Republic of Macedonia, 2002
| Symptom/disease | One smoker in householda | ≥2 smokers in householda | ||
| OR (95% CI) | aOR (95% CI) | OR (95% CI) | aOR (95% CI) | |
| Current wheeze | 1.06 (0.75–1.48) | 1.04 (0.74–1.48) | 1.31 (0.95–1.80) | 1.20 (0.86–1.68) |
| Current speech-limiting wheeze | 1.53 (0.62–3.81) | 1.19 (0.47–3.05) | 1.49 (0.61–3.67) | 1.12 (0.44–2.86) |
| Current dry night cough | 1.12 (0.87–1.45) | 1.07 (0.82–1.40) | 1.34 (1.05–1.72) | 1.20 (0.93–1.55) |
| Ever-diagnosed asthma | 2.06 (0.95–4.45) | 2.01 (0.88–4.59) | 1.47 (0.66–3.27) | 1.63 (0.70–3.79) |
| Current nose and eye symptoms | 1.23 (0.83–1.81) | 1.11 (0.75–1.66) | 0.94 (0.63–1.41) | 0.76 (0.50–1.16) |
| Current severe nose symptoms | 1.31 (0.71–2.39) | 1.16 (0.62–2.15) | 1.23 (0.67–2.24) | 1.03 (0.55–1.92) |
| Ever-diagnosed hay fever | 1.31 (0.90–1.91) | 1.24 (0.85–1.82) | 1.19 (0.82–1.73) | 1.10 (0.75–1.61) |
| Current symptoms of eczema | 1.27 (0.72–2.22) | 1.09 (0.61–1.95) | 1.01 (0.57–1.79) | 0.84 (0.46–1.54) |
| Current severe eczema symptoms | 1.11 (0.44–2.84) | 0.92 (0.34–2.53) | 0.69 (0.26–1.82) | 0.63 (0.21–1.83) |
| Ever-diagnosed eczema | 1.36 (0.84–2.23) | 1.46 (0.88–2.42) | 1.07 (0.65–1.77) | 1.07 (0.63–1.82) |
OR=Odds ratio; aOR=adjusted OR; CI=confidence interval; Current=in the last 12 months.
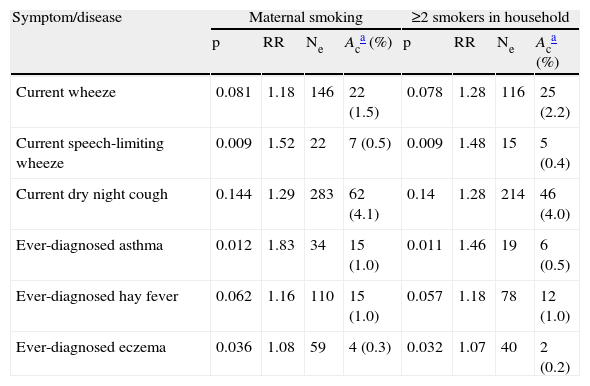
Stepwise calculations of the number of attributable cases of current and ever-diagnosed asthma, and ever-diagnosed hay fever and eczema in relation to maternal smoking and ≥2 smokers in household are shown in Table 4.
Stepwise calculation of the number of asthma, hay fever, and eczema cases attributable to maternal smoking and ≥2 smokers in household in 3026 children aged 13–14 yrs in Skopje, Republic of Macedonia, 2002
| Symptom/disease | Maternal smoking | ≥2 smokers in household | ||||||
| p | RR | Ne | Aca (%) | p | RR | Ne | Aca (%) | |
| Current wheeze | 0.081 | 1.18 | 146 | 22 (1.5) | 0.078 | 1.28 | 116 | 25 (2.2) |
| Current speech-limiting wheeze | 0.009 | 1.52 | 22 | 7 (0.5) | 0.009 | 1.48 | 15 | 5 (0.4) |
| Current dry night cough | 0.144 | 1.29 | 283 | 62 (4.1) | 0.14 | 1.28 | 214 | 46 (4.0) |
| Ever-diagnosed asthma | 0.012 | 1.83 | 34 | 15 (1.0) | 0.011 | 1.46 | 19 | 6 (0.5) |
| Ever-diagnosed hay fever | 0.062 | 1.16 | 110 | 15 (1.0) | 0.057 | 1.18 | 78 | 12 (1.0) |
| Ever-diagnosed eczema | 0.036 | 1.08 | 59 | 4 (0.3) | 0.032 | 1.07 | 40 | 2 (0.2) |
p=outcome incidence in the unexposed group; RR=Relative risk; Ne=number of cases exposed to maternal or ≥2 smokers in household; Ac=number of attributable cases; Current=in the last 12 months.
The much lower prevalence of ever-diagnosed asthma in contrast to the prevalence rates of current wheeze and dry night cough apart from chest infection suggests under-diagnosis of asthma and/or underreporting of the diagnosis by the young adolescents in our country, which can be noted in other less affluent countries worldwide.13 In contrast, hay fever and eczema seem to be over-diagnosed and/or over-reported.
The results of the present study demonstrated an increased risk of current night dry cough apart from chest infection in relation to maternal smoking habit only. In the asthma questionnaire, this kind of cough was considered as a current asthma symptom. However, as the prevalence of current dry night cough was much higher in contrast to those of current wheeze and ever-diagnosed asthma, it seems that cough due not only to asthma was also considered in the participant’s responses. The positive association present in relation to maternal, but not paternal and household smoking habits may be explained by the considerably greater time children usually spend in the company of their mothers. Maternal smoking is considered to have a dominant impact on a child’s health with little effect of paternal smoking.2 Some active smoking in the investigated age group cannot be ruled out as the smoking habit of the family tends to influence the smoking behaviour of young adolescents.11
The number of smokers in the household did not show a significant association with asthma, rhinitis, and eczema. Related to the relatively safe environment and sufficient number of closely located playgrounds in Skopje, our young adolescents might spend more time outside their homes compared to their younger siblings. On the other hand, upon a diagnosis of asthma or rhinitis or eczema in the respondents, parents might quit or reduce smoking at home despite their tobacco smoke habits, which may influence the established results.
The estimation of attributable cases of current and ever-diagnosed asthma, ever-diagnosed hay fever and eczema did not show a notable impact by home ETS exposure, except for night dry cough. In the absence of maternal smoking or two or more smokers in household, there would be 4.1% or 4% fewer young adolescents with night dry cough. In this calculation we did not combine asthma parameters because of the supposed under-diagnosis of asthma.
We cannot exclude information bias with the use of questionnaires. The information of the length of smoking habits and the number of cigarettes smoked daily by household smokers, active smoking habit of the respondents and familiar atopy, which can alter our results, were not considered in the present study as we have not modified the ISAAC Phase Three questionnaires for 13–14 year-olds. However, since it was the young adolescents themselves who completed the questionnaires, they would probably not have answered these questions correctly. On account of the almost equal gender distribution and prevalence rates of parental smoking habits and number of smokers in the family, the analyses were conducted on the whole group of respondents with gender included as a controlling factor.
Our findings concur with the results reported in many other studies.6,9,24,25 Moyes et al.,9 using ISAAC questionnaires, have documented an increased risk of current night cough and no other asthma symptom, and an increased risk of nasal symptoms in young adolescents from smoking households in New Zealand. Raherison et al.,6 in a large population-based sample of schoolchildren living in metropolitan France, also did not find an association between current exposure to ETS at home and current asthma, rhinitis, eczema, and atopy. Postnatal ETS, through the number of smokers in the household, was not independently associated with asthma in a study conducted by Li et al.24 Also consistent with the results established in the present study are results found in other studies carried out on young adolescents11,12,26 in contrast to the findings in younger age groups.8,11,27 This age-related decline in the strength of ETS effect is considered to reflect the diminishing level of exposure in the household with increasing age and/or the differential effects on the incidence of various forms of wheeze illness with a stronger influence on viral associated wheeze common in early childhood, and a weaker association with atopic wheeze that is often of later onset.2
In contrast, Tanaka et al.12 using ISAAC questionnaires in Japan, have found that current heavy passive smoking in the household was related to an increased prevalence of wheeze and asthma, especially in younger children and children with a positive parental allergic history. Comparing the parental smoking behaviour and its effects in Finland and Russia, Hugg et al.28 have demonstrated a related risk of asthma to high current maternal smoking in Finnish schoolchildren, in contrast to Russian schoolchildren, in whom the risk of allergic conjunctivitis was increased. In a PATY study, strong evidence linking current smokers in household to wheeze, asthma and nocturnal cough, and inverse effect estimates for hay fever were found in 6–12 year-olds.17 The presence of a mother who smokes was also positively associated with asthma among children aged 13–14 years in a questionnaire-based ISAAC study in Rio de Janeiro.29 Using the same questionnaire among the same age group, we also found a positive association between maternal smoking and asthma, but the significance disappeared after adjustment for confounders.
The established results in the present study are probably due to the older investigated age group, which has been described as having a smaller risk of asthma caused by ETS than pre-school children.2,5 A relatively safe environment in Skopje enables young adolescents to be outdoors much of the time and to avoid a great household tobacco smoke exposure. The high intake of dietary antioxidants and low exposure to other than tobacco smoke indoor inhaled oxidants, which were documented in our respondents, may also contribute to our findings (decreased adjusted ORs compared to crude ORs). It is unlikely that the under-diagnosis of asthma and/or underreporting of the diagnosis by the young adolescents influence the established results as current asthma symptoms were also examined in relation to ETS, and on the other hand, hay fever and eczema were observed to be over-diagnosed and/or over-reported. Finally, there is a possibility that household smoking in adolescence really does not have a significant impact on the investigated diseases, bearing in mind that in our country their prevalence rates are low despite the very high prevalence of ETS in household.
In conclusion, in this questionnaire-based cross-sectional study on young adolescents in The Republic of Macedonia, a country with low prevalence rates of asthma, rhinitis and eczema and high household tobacco smoke exposure, the only observed adverse effect of household tobacco smoke was related to night cough and maternal smoking habits. Nevertheless, due to the broad spectrum of harmful effects which have been already documented in children by second-hand tobacco smoke, counselling parents to quit smoking remains a public health policy of high priority.
The authors would like to thank children for their participation, and the principals, psychologists, and teachers for their collaboration in the survey. The Ministry of Education and Science of The Republic of Macedonia provided financial support for the study.