The Th1/Th2 balance has not been characterized in patients suffering from flea bite-induced papular urticaria (FBPU). Our aim was to improve understanding of the immunopathogenesis of CD4+and CD8+T-cells in humans suffering from flea bite-induced papular urticaria.
MethodsPeripheral blood mononuclear cells were obtained from 18 pediatric patients and 10 age-matched healthy controls. Cellular phenotypes, intracellular production of interferon gamma (IFNγ) and interleukin-4 (IL-4) in T-cells stimulated with polyclonal stimuli was determined by flow cytometry following short-term in vitro stimulation.
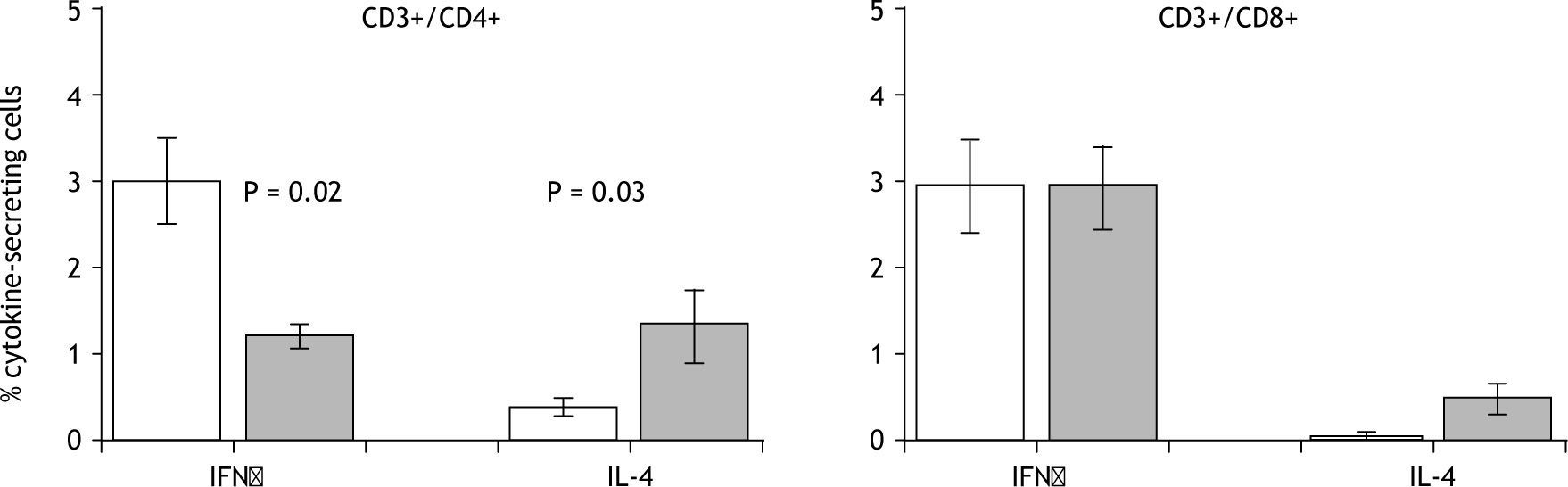
ResultsThe results revealed lower frequencies of IFNγ-secreting (p=0.02) and higher frequencies of IL-4-secreting (p=0.03) CD4+T-cells in patient lymphocyte cultures compared to healthy control cultures in the presence of polyclonal stimuli. This is the first description of differential cytokine patterns in papular urticaria patients.
ConclusionPatients suffering from papular urticaria have an atopic status compared to healthy children.
Allergic diseases are characterized by a complex immune response that is predominantly related to the secretion of Th2-associated cytokines. These cytokines are responsible for inducing and maintaining allergic inflammation1,2.
Papular urticaria has been characterized as a chronic allergic disease, manifesting itself as hypersensitive reactions on the skin caused by exposure to ectoparasites, such as fleas3. Previous studies concerning the immunological mechanisms involved in this disease have shown large eosinophil infiltrates in biopsies from patients' cutaneous lesions4. Moreover, it has been shown that simultaneous exposure of patients' dendritic cells to flea antigens and lipopolysaccharide induces increased expression of the CD86 co-stimulating molecule that is associated with the induction of a Th2 response5. Humoral immunity studies have revealed greater protein recognition in flea extract by IgG1 and IgG3 from healthy individuals and by IgE from patients with papular urticaria (unpublished data).
The presence of eosinophils in skin lesions and differential antigenic recognition by some antibody isotypes led to the hypothesis that patients suffering from flea bite-induced papular urticaria (FBPU) would have a Th2-dominant response, as has been described for other allergic diseases. The objective of the current work was to identify cytokines secreted by CD4+ and CD8+ lymphocytes (IFNγ and IL-4) as indicators of Th1 and Th2 responses, respectively, after stimulation with a polyclonal stimuli.
Materials and MethodsStudy populationThe sample included 18 patients, aged one to 13, who had been clinically diagnosed as suffering from FBPU. These patients were attended by the Pediatric Dermatology and Allergy Department of the Fundación Santa Fe de Bogotá, in Bogotá, Colombia. Patients excluded from consideration for participation in the study included those having secondary infected lesions or suffering from immuno-suppression caused by systemic disease, as well as those who had been treated with immuno-suppressive medication; those who had received antihistamines 5days before the consultation, and/or who had been treated with flea extract. Ten healthy children admitted to the same institution for elective surgery and sharing the same age group and socioeconomic characteristics as the FBPU patients were included as controls. The investigation was approved by the ethics committees at both the Fundación Santa Fe de Bogotá and the Universidad Javeriana.
Disease diagnosisFBPU was diagnosed according to clinical characteristics. Patients usually had lesions appearing as grouped papules, a symptom commonly associated with pruritus. Papules were often excoriated or crusted, appearing intermittently in a chronic course and leaving hypo- or hyper-pigmented macules behind. These were most often located in areas where clothing fits snugly such as the socks and the waist-band. In some patients, exposed areas of the extremities were also affected.
PBMC isolation and lymphocyte stimulationPeripheral blood mononuclear cells (PBMC) were prepared from fresh heparinised blood using a Ficoll-hypaque density gradient (Sigma, St. Louis, MO). The cells were adjusted to 2×106 cells in a final 2ml volume of RPMI 1640 medium containing antibiotics, non-essential amino acids, sodium pyruvate, and 10 % FCS. PBMC were incubated with 1μg/ml of anti-CD28 and anti-CD49d. A positive control of 3.7μg/ml of staphylococcal enterotoxin B (SEB) was used6. A negative control (no stimulus) was included in every experiment. The cultures were incubated for 9h at 37°C in 5 % CO2, followed by an additional 3h incubation in the presence of 10μg/ml Brefeldin A (Sigma). Surface staining was performed with anti-CD3-FITC, anti-CD4-PerCP or anti-CD8-PerCP, anti-CD69-APC (BD Pharmingen); after fixation and permeabilisation, the cells were then stained with IFNγ-PE or IL-4-PE (BD Pharmigen). Data were acquired and analysed using a FACS-Calibur flow cytometer (BD Immunocytometry Systems) and Cell Quest software.
Quantifying cytokine levels in plasmaCytokines were quantified in plasma using a Cytometric Bead Array kit (BD Biosciences), following the manufacturer's instructions. Beads displaying different PerCP fluorescence intensities were used for the quantification; they were coated with PE-conjugated capture antibodies that were specific for IFNγ and IL-4. Concentration was calculated using different cytokine patterns at known concentrations. Data were acquired using a FACSCalibur flow cytometer (BD Immunocytometry Systems), and Cell Quest software was used for analysis.
Statistical analysisSPS statistical software was used for analysing the data. All categorical variables were described using frequencies, while continuous variables were reported as means or medians with their corresponding 95 % confidence intervals and standard deviations. The U Mann–Whitney test was used for making comparisons; the significance level was set at < 0.05.
ResultsPBMC were obtained and cultured in the presence of polyclonal stimuli to characterize the Th1/Th2 cytokine balance in lymphocytes from healthy controls and paediatric patients suffering from FBPU.
The patients were 50/50 % with respect to sex, the age average was 3.5years (SEM 0.7years). Twenty-eight percent of the patients in this study reported a personal history of atopy (asthma, allergic rhinitis, or atopic dermatitis) while 78 % of them reported a family history of atopy. Seventy percent of healthy children were males and the age average was 3.4years (SEM 0.6years); none reported a personal history of atopy, and 11 % reported a family history of atopy.
Plasma levels of IFNγ and IL-4 were quantified by flow cytometry to establish differences in plasmatic cytokine levels between the patients and controls. The results (32.7pg/ml, SEM 3.3pg/ml in healthy children and 29.5pg/ml, SEM 2.5pg/ml in patients for IFNγ and 45.6pg/ml, SEM 3.6pg/ ml in healthy children and 55.8pg/ml, SEM 2.7pg/ml for IL-4) revealed no quantitatively significant differences between patients and healthy controls for any of the cytokines evaluated.
Expression levels of cellular activation markers were examined in CD3+/CD4+ and CD3+/CD8+ T-cells to assess the activation status of the T-cells after various culture conditions. When cells were unstimulated or activated with SEB as a polyclonal stimulus, CD69 expression levels were similar in both patients and controls (24.2 % in healthy children and 29.3 % in patients).
Polyclonally stimulated CD3+/CD4+/CD69+ or CD3+/CD8+/CD69+ cells were analyzed to identify IFNγand IL-4-producing cells in both the patient and the control group. A greater percentage of IL-4-secreting CD4+ T-cells were found in patients (1.3 %) compared with healthy children (0.4 %) (p = 0.03), while a greater proportion of IFNγ-secreting CD4+ T-cells were found in healthy controls (3 %) compared with patients (1.2 %) (p = 0.02). No differences were found in IL-4- or IFNγ-secreting CD8+ T-cells (fig. 1).
DiscussionFBPU in humans has classically been defined as a chronic allergic disease. However, no reports have been published to date indicating whether this pathology is related to a Th2-predominant response. IFNγ and IL-4 secreted by lymphoid subpopulations using polyclonal stimuli have been identified in this work, as well as plasmatic cytokine levels as indicators of Th1 and Th2.
Previous studies have reported greater circulating levels of IL-4 in allergic individuals compared to healthy controls7-9. Plasmatic levels of IL-4 and IFNγ were quantified in patients suffering from FBPU to establish whether the differences found at cellular level were reflected at the systemic level. According to our results, no significant difference was found between the patients and healthy controls with regard to plasmatic IL-4 and IFNγ levels. These results indicate that the disease's clinical manifestations were not associated with alterations at the systemic level.
CD69 expression was evaluated to establish whether allergic inflammation in papular urticaria was associated with functional alterations at the cellular level; CD69 has been used as both an in vivo and in vitro activation marker10. Lymphocytes from patients suffering from hypersensitive reactions to drugs have increased CD69 expression as compared to healthy individuals' lymphocytes11,12, indicating that their functional activity is important. The results shown here indicate that both cells from patients suffering from FBPU and those from healthy controls were equally competent because no differences in CD69 activation marker expression were found after polyclonal stimulation.
With regard to the balance between Th2 and Th1 cytokine production in allergic diseases, it has been shown that children at risk for developing atopic dermatitis (followed-up from birth to 3years of age) are predominantly predisposed to respond with Th2 cytokine production after SEB stimulation13. Quantification of PBMC-secreted cytokines from children suffering from asthma, eczema, and/or atopic rhinitis has revealed increased IL-4 and IL-5 production and reduced IFNγ production after SEB stimulation as compared to cells from healthy children14.
The evaluation of Th2 and Th1 cytokines in this study through the quantification of IL-4 and IFNγ production (after polyclonal stimulation) demonstrates that cells from patients suffering from papular urticaria predominantly responded with Th2 cytokine production, indicating that patients underwent an atopic stage that may have predisposed them to develop the disease. A tendency towards greater IL-4 secretion was observed in the case of CD8+ T-cells, even though the difference was not significant. Differential cytokine secretion patterns by T-cells may be important contributing factors in the development of childhood papular urticaria.
These results are similar to those reported in the canine model of the disease15. Flea bite-induced allergic dermatitis is one of the most common allergic diseases in dogs, corresponding to around 50 % of canine dermatological disorders. This allergic dermatitis generally coexists with other allergic conditions. Examination of peripheral blood cell-secreted cytokines in the canine model by mRNA quantification revealed greater mRNA expression for IL-4 in allergic dogs and increased mRNA expression for all cytokines quantified (including IFNγ) following exposure to fleabite15.
These findings support the hypothesis that susceptibility for the development of FBPU is related to a base atopic state and the predomination of Th2 cytokine production (such as IL-4).
This is the first description of differential cytokine patterns in papular urticaria patients.
Reported variations in the regulation of an allergic response to flea antigens during the development of papular urticaria could facilitate the implementation of antigen-specific therapies favoring allergy desensitisation.
Funding/SupportThis study was financed by Fundación Santa Fe de Bogotá and the Vicerrectoría Académica, Pontificia Universidad Javeriana, Bogotá, Colombia.
The authors wish to thank the collaboration of doctors Manuel Forero, Mariela Tavera, Clara Ordoñez, Ana María Salazar, Armando Rojas, and Adriana Motta for help to get the patients.