INTRODUCTION
Latex sensitization is recognized as a serious health problem since latex products are increasingly used, especially among health care workers and patients undergoing multiple surgical interventions, such as spina bifida patients 1-5. Some epidemiological studies have reported that the incidence and prevalence of latex allergy has increased during the past 10 years, and demonstrated a prevalence ranging between 2.9 % and 17 % in health care workers 6,7. For the latter risk group there are numerous routes of exposure such as the skin, from direct contact with latex products, and/or the nose and bronchi, from inhalation of aerosolized glove powder coated with natural rubber latex allergens. Various studies 8-10 have reported several risk factors for the development of latex allergy, such as having spina bifida, being subjected to multiple surgical interventions, having a history of atopy and/or food allergies namely to kiwi fruit, banana, avocado, tomato, papaya and chestnut.
Natural rubber latex is a complex mixture of several substances such as rubber particles (30-40 %), proteins (2,3 %) and water (55-65 %). In manufactured products, proteins can comprise up to 3 % of the contents 11,12.
Latex sensitization, especially among health care workers, is linked to proteins from natural rubber latex gloves. Several hundred proteins have been described in latex, of which 13 (Hev b 1 to Hev b 13) have been recognized by the International Union of Immunological Societies (IUIS) as latex allergens 13. From these proteins, Hev b 1, Hev b 3 and Hev b 7 are major allergens for spina bifida patients, and Hev b 2, Hev b 5, Hev b 6.01 and Hev b 13 are major allergens for healthcare workers. The reason for this discrepancy in the allergen sensitisation profiles is currently unknown 14-17. However, although genetic factors may influence this differential pattern of allergen recognition in these risk groups, the different reactivity patterns may also be due to differences in the allergen make-up of internal and external glove surfaces. Therefore, the purpose of the present study was to characterise and identify latex allergens in the internal and external surfaces of natural rubber latex surgical gloves.
MATERIALS & METHODS
Characterization of latex-allergic patients
Two health care workers with a clinical history of latex allergy were recruited. One (A) had a history of rhinoconjunctivitis and bronchial asthma symptoms on exposure to latex-rich environments whereas the second patient (B) had a history of anaphylactic shock upon contact with latex allergens. Sensitisation to latex allergens was confirmed by positive skin prick tests (Bial-Aristegui), as well as positive latex-specific IgE results. One of the patients had positive latex-specific IgE to Hev b 5 (12.4 kUA/l), Hev b 6.01 (9.17 kUA/l), and Hev b 6.02 (9.14 kUA/l), whereas the second patient had a reactivity of 1.70 kUA/l, 1.02 kUA/l and 1.12 kUA/l, respectively to the same allergens. Sera was obtained from both of these patients.
Latex allergen extraction
Latex allergens were extracted separately from the internal and external surfaces of two commercial brands and batches of latex surgical gloves. Extracts were separately obtained from both surfaces according to the method of Tomazic et al 23, and recommended by the American Society for Testing and Materials (ASTM D 5712-95, 1995). Briefly, each glove was sealed with a string in the proximal wrist end, covered with 200 mL of phosphate-buffered saline (PBS), in a 1L Erlenmeyer flask under agitation (260 r.p.m.) at 37 °C for 24 h. Extracts of the internal glove surface were prepared in the same way after turning the internal surface out. Glove extracts were centrifuged at 4000 g for 30 min to remove insoluble particles, concentrated (AMICON 8050 unit, Bedford, U.S.A.) with a regenerated cellulose membrane (NMWL 1000 Da, Millipore, Bedford, U.S.A.), dialyzed (snakeskin pleated tube, Pierce, Rockford, U.S.A., with NMWL 3500 Da), lyophilized and stored at 18 °C.
SDS-PAGE assays
Thirty-microlitre aliquots of each extract were separated by analytical 15 % or 4-20 % gradient SDS-PAGE. A Rainbow Marker (Amersham, UK) was used as a molecular weight standard. Proteins were visualized by Coomassie Blue staining.
IgE immunoblotting
Thirty microlitre aliquots of each glove extract were separated by preparative 15 % SDS-PAGE. Proteins were blotted onto PVDF membrane (Hybond-P- Pharmacia, Buckinghamshire, England). PVDF strips of 5 mm were blocked twice for 5 min and once for 30 min in buffer (50 mmol/L sodium phosphate, pH 7.5, 0.5 % v/v TWEEN-20, 0,5 % w/v BSA, 0.05 % w/v NaN3). PVDF strips containing blotted latex extracts were incubated with sera diluted 1:10 in buffer or with buffer alone, overnight at 4 °C. After two washes in buffer for 5 min and one for 30 min, bound IgE was detected using alkaline phosphatase-labelled antihuman IgE antibodies (Sigma, Steinheim, Germany) diluted 1:1000 in buffer, for 2 h, at room temperature. The reaction was visualised using enhanced chemifluorescence analysis, ECF (Amersham Biosciences, Buckinghamshire, England). Strips were washed as above, dried and exposed to Molecular Imager Fx Pro System (BioRad, Hercules, U.S.A.)
Immunoenzymatic assays (ELISA)
ELISA analysis for Hev b 1, Hev b 3, Hev b 5 and Hev b 6.02 was performed using a commercial kit (Fitkit, Fit Biotech Oyj Plc, Finland) according to the manufacturer instructions. Results were obtained through a collaboration with this institution.
Chromatographic method
Hydrophobic Interaction Chromatography (HIC) was performed using a FPLC system (Amersham-Pharmacia, Uppsala, Sweden) on a Sepharose CL-6B column modified with butyl-1,4-bis-(2,3-epoxy-propoxyd) 19. The gel was packed in a column XK 16/20 (Amersham Pharmacia Biotech, Uppsala, Sweden) and equilibrated with PBS 0,01M, with ammonium sulphate, 1.0 M, pH 7,4, at room temperature (25 °C). Fractions (2 ml) were collected and protein concentration was determined using the Bradford method 20.
RESULTS
HIC assays
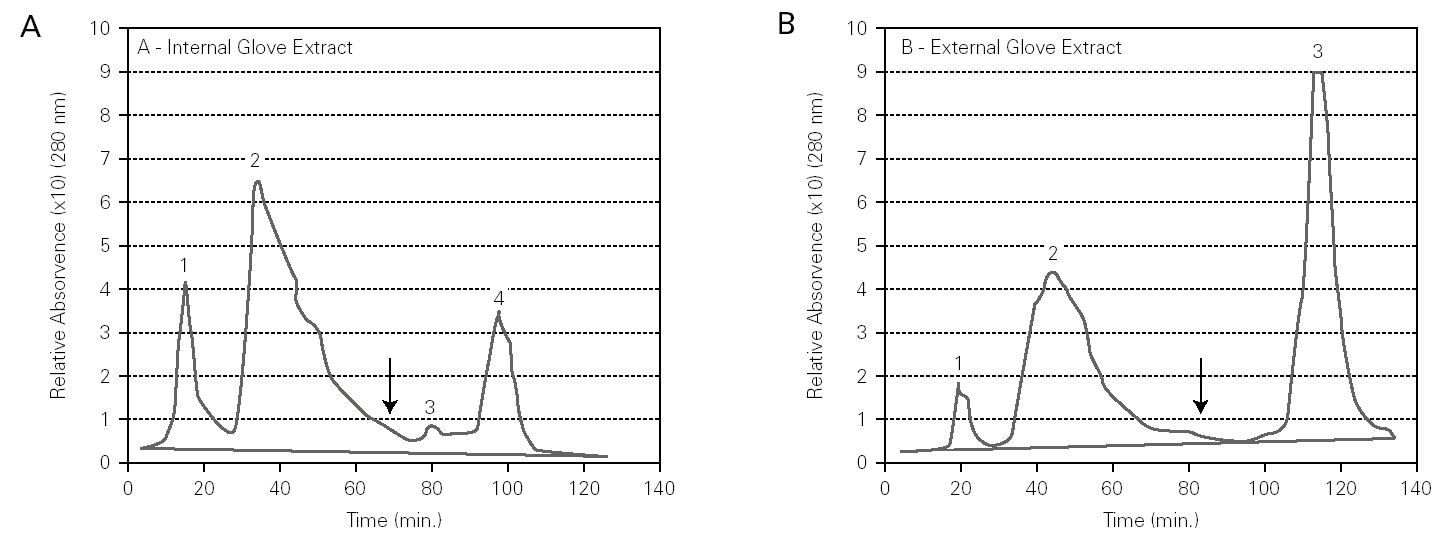
Chromatographic profiles of internal and external glove surfaces showed both qualitative and quantitative differences in protein content (fig. 1). Firstly, the internal surface extracts had a higher content in total proteins than the external surface extracts. Secondly, the internal surface extract had higher amounts of hydrophilic proteins (peaks 1 and 2) than the external surface extracts, which had mainly hydrophobic proteins (peak 3).
Figure 1.--HIC assays on Sepharose CL-6B modified with butyl-1,4-bis-(2,3-epoxy-propoxyde), for internal (A) and external (B) surfaces extracts of latex gloves. Buffer: PBS 0.01 M, pH 7.4 containing 1.0 M ammonium sulphate. Elution (↓) was obtained with PBS 0.01 M, pH 7.4. Sample: 100 μl (0,5 mg protein/ml).
SDS-PAGE and IgE immunoblotting assays
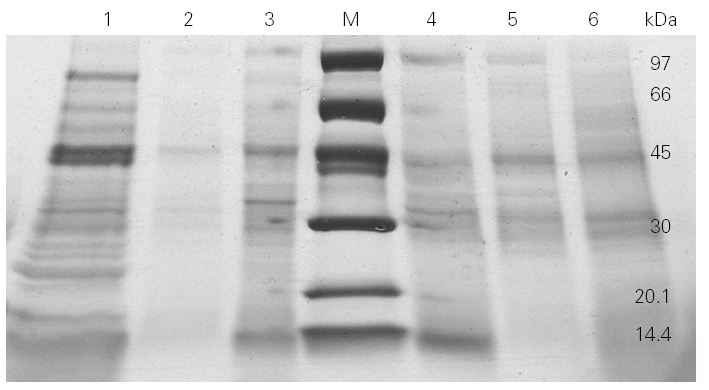
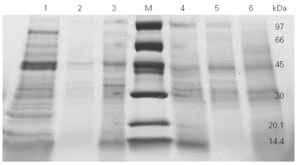
We then went on to analyse the protein and allergen content of the glove extracts from two different brands. SDS-PAGE assays (fig. 2) showed a differential protein composition between internal and external surfaces of the gloves that was seen within each brand. The internal surface had a wider range of proteins of varying molecular weights, whereas the external surface showed fewer bands of low molecular weight proteins.
Figure 2.--4-20 % SDS-PAGE gradient gel of total (lines 1 and 6), internal (lines 3 and 4) and external (lines 2 and 5) extracts from two different brands (brand X: lines 1, 2 and 3; brand Y: lines 4, 5 and 6) of surgical latex gloves.
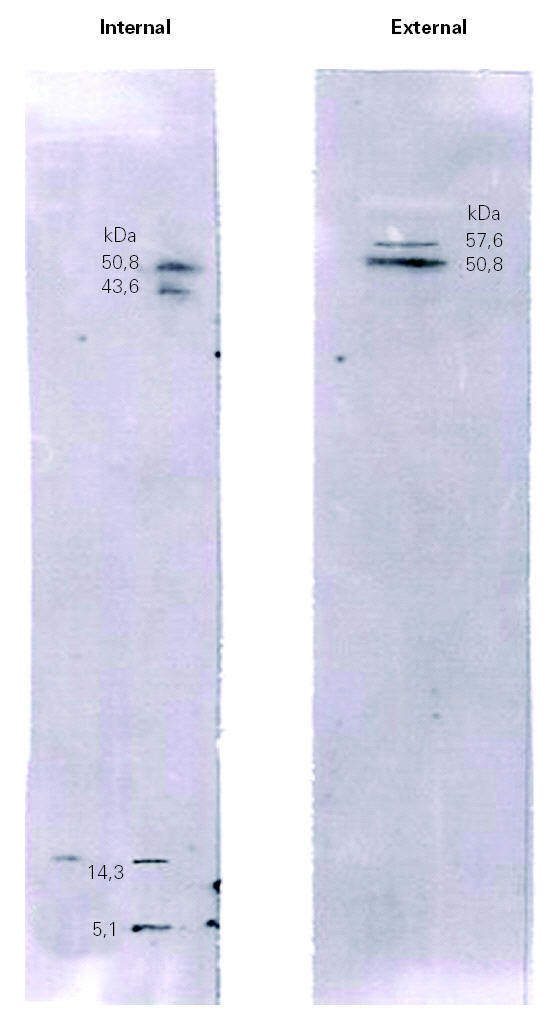
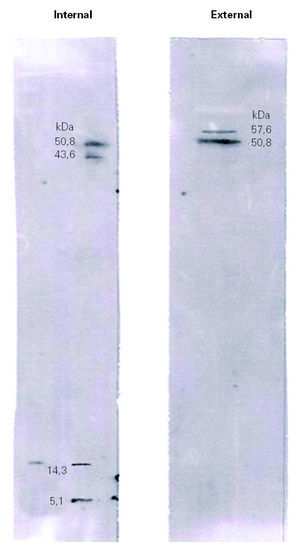
Immunoblotting assays showed that many of the proteins visualized by Coomassie Blue staining failed to react with IgE antibodies (fig. 3).
Figure 3.--Immunoblotting of the internal and external surfaces of natural rubber latex surgical gloves, with serum of a health care worker patient (A).
Immunoblotting of the internal surface showed four bands with molecular weights of 50.8 kDa, 43.8 kDa, 14.3 kDa and 5.1 kDa. Immunoblotting of the external surface showed two bands, at 57.6 kDa and 50.8 kDa.
Immunoenzymatic assays (ELISA)
Glove brands were analysed by IgE ELISA (table I), which showed differential localisation patterns of latex allergens, since the internal glove surface had high amounts of Hev b 5 and Hev b 6.02 whereas the external glove surface had a high content in Hev b 1, Hev b 3 and Hev b 6.02.
DISCUSSION
The current study demonstrated the occurrence of differences in the allergen profile between the internal and external surfaces of latex surgical gloves. The study also showed that different glove brands and batches contained widely varying protein and allergen contents.
HIC is a powerful chromatography technique that takes advantage of hydrophobicity of proteins, promoting its separation based on hydrophobic interactions between immobilized hydrophobic ligands on the gel and non polar regions on the surface of proteins 19. In our study, the HIC method showed differences in the chromatographic profiles of internal and external latex glove surfaces (fig. 1). These differences were visualized both at a qualitative and a quantitative level by the size and number of peaks on the chromatographic profiles. The internal surface extract had higher amounts of hydrophilic proteins (fig. 1A, peaks 1 and 2) than the external surface extracts, which had mainly hydrophobic proteins (fig. 1B, peak 3). According to D'Auzac et al 22, the major latex allergens for health care workers, Hev b 2, Hev b 5, have mainly hydrophilic characteristics whereas Hev b 1 and Hev b 3, the major allergens for spina bifida patients, are mainly hydrophobic. Thus, our HIC results, showing that the internal surface of surgical latex gloves was rich in hydrophilic proteins, may suggest that these correspond to major latex allergens for health care workers. On the other hand, the external surface had mainly hydrophobic proteins that may include major latex allergens for spina bifida patients.
SDS-PAGE analysis showed different protein profile between internal and external surfaces (fig. 2).
The results obtained by IgE immunoblotting of patient A (fig. 3) and patient B (data not shown) showed a differential reactivity of sera with the internal and external extracts of latex gloves. This result revealed a different allergenic profile between both surfaces of surgical latex gloves. When comparing immunoblotting with SDS-PAGE results (figs. 2 and 3), it became apparent that many of the proteins visualized by Coomassie Blue staining failed to react with IgE antibodies, which suggests that many of the extractable proteins do not represent latex allergens. Thus the allergenic components do not seem to constitute the major fraction of the total proteins extractable, a result that is in agreement with those from Mahler et al 21. The identification of bands obtained by immunoblotting analysis was carried out by molecular weight comparison. Immunoblotting of the internal surface showed four bands with molecular weights of 50.8 kDa, 43.8 kDa, 14.3 kDa and 5.1 kDa. The 50.8 kDa band might correspond to Hev b 9, the 43.8 kDa band might correspond to Hev b 7.01, Hev b 7.02 or Hev b 13, the 14.3 kDa band might correspond to Hev b 6.03 or Hev b 8, and the 5.1 kDa band might correspond to Hev b 6.02. Immunoblotting of the external surface showed two bands, at 57.6 kDa and 50.8 kDa, with the former probably corresponding to Hev b 1 and the latter to Hev b 9. The ambiguity in the identification of these bands should be resolved using allergen patterns.
We have demonstrated by ELISA methods, and with the four antibodies available, that the internal glove surfaces contain Hev b 5, major allergen in health care workers risk group, as well as, Hev b 6.02. On the other hand, the external surfaces of latex gloves showed Hev b 1 and Hev b 3, which are major allergens for spina bifida patients, as well as Hev b 6.02. Curiously, no major allergens for health care workers were detected on the external surface of latex surgical gloves.
These two risk groups became sensitized to latex by different routes of exposure. In fact, health care workers directly contact with the internal surfaces of latex gloves and may inhale aerosolized glove powder coated with natural rubber latex allergens that contact with the mucous membrane of the nose, throat and airways of the lung 23-30. On the other hand, spina bifida patients may became sensitized by contact with the external surface of latex gloves during surgical procedures 31. Differences in the allergen makeup of internal and external surgical glove surfaces may result from manufacture procedure Yip and Cacioli 32. In fact, the method of manufacture including the means used to coat gloves to make donning easy, can influence the eventual exposure to latex allergens on the latex glove. In several manufacturing processes of latex gloves, during the vulcanization process, the water trapped in the latex film is turned into steam and escapes through it. As the steam pass on the film carry residual nitrates and soluble proteins 26. Consequently, the surface of the latex glove that is on the outside of the moud (which will be the internal surface when the glove is donned) is rich in these materials.
The analysis by the Bradford method showed a higher content in total proteins in internal (0.40 mg/g glove) than in external surface extracts (0.13 mg/g glove), which is in agreement with results obtained by Mahler et al 21. Since the internal surfaces had most of the extractable latex allergens, our study may explain the high rate of cutaneous manifestations observed in latex-allergic health care workers. Our findings also demonstrate that allergens from the internal surfaces of latex surgical gloves are different from the external ones.
In summary, our results showed substantial differences in the composition of latex allergen profiles between the internal and external surfaces of latex surgical gloves, which may suggest a relationship between the localization of latex allergen and sensitisation routes in different risk groups for latex allergy. Our study may, at least in part, explain the different allergen sensitization profiles observed between health care workers and spina bifida patients. Our novel findings will be further evaluated by investigation of spina bifida patients as well as, a greater number of health care workers.
Correspondence:
Prof. L. Taborda-Barata, MD
Department of Medical Sciences
Faculty of Health Sciences. University of Beira Interior
Avda. Marquês d'Ávila e Bolama.
6200-001 Covilhã. Portugal.
E-mail: tabordabarata@ubi.pt