Cow’s milk protein allergy (CMPA) is the most common type of food-allergy in younger children. Prognosis is usually good, with most children developing tolerance before school age. Children may present with a wide spectrum of symptoms that range from mild to severe; skin reactions such as angioedema and urticaria and gastrointestinal symptoms are the most common presentations of CMPA. Approximately one-third of CMPA patients suffer from multiple food-allergies; severe conditions such as anaphylactic shock (9%), eosinophilic esophagitis (4.7%), and food-protein induced enterocolitis (1%) may also develop in some children. Timely and accurate diagnosis and management is essential for proper growth and development of children with CMPA. In this expert consensus report, we aimed to adapt current understandings in the CMPA field to the specific conditions in Turkey and health system to help physicians with their day-to-day decision making.
Food-allergy is defined as an adverse immune reaction in response to exposure to a specific type of food. Recent studies indicate a rise in the prevalence of food-allergy, affecting up to 10% of the population, particularly in the industrialized areas.1 In Turkey, self-reported food-allergy prevalence in adult and adolescent populations were determined as 9.5% and 11%, although proven food-allergy was detected in 0.3% and 0.15% after clinical investigations.2,3
Cow’s milk protein (CMP) is one of the earliest allergens in an infant’s life. CMP allergy (CMPA) is an immune-mediated hypersensitivity reaction to proteins in cow’s milk, mainly to casein and β-lactoglobulin.4 It is a pediatric allergic disease that resolves in almost all cases before adult life. CMPA may present with a variety of specific and nonspecific symptoms and should not be confused with lactose intolerance, the inability to digest lactose. Clinical manifestations vary according to the type of immune reaction elicited (IgE-mediated, non-IgE-mediated, mixed), sensitivity to allergen, and age of the patient, posing important diagnostic challenges for physicians.5 Accurate and timely diagnosis, optimal management, as well as measures to prevent food disease are important for normal growth and development and minimize the burden on the healthcare system.
Several international guidelines have reviewed the existing literature and proposed specific recommendations for diagnosis, management, and prevention of CMPA. However, since food-allergy and its management are largely affected by social contexts, eating habits, and available resources, guidelines such as BSACI, DRACMA and ESPGHAN encourage the development of region-specific guidelines that would suit the needs of children from all social classes in the countries they are intended for.6–9
Turkey is a large country situated between Europe and Asia. Because of geographic barriers, population density differences, and sociocultural variations, access to healthcare is not homogeneous across Turkey. As a country of increasing population growth and high birth rates, pediatrics is one of the medical specialties most sought after. Anecdotal evidence suggests that in some crowded centers pediatricians may be seeing too many patients per day.10 Similarly, in primary care centers, which is usually the first healthcare center families visit for their infant in distress, physicians may have less than five minutes which includes taking history, performing physical exam and prescribing or administering treatment.11 In this context, guidelines play an important role in helping the pediatrician with the best and safest course of action in complex and multi-faceted diseases such as food-allergies. It is our belief that a local guideline targeting to prioritize local characteristics of Turkey and healthcare setting is needed to optimize CMPA diagnosis and management on patients and physicians alike.
In this expert opinion statement, we intended to review the current guidelines and literature and come up with practical recommendations for CMPA diagnosis and management in Turkey, aimed particularly at pediatricians in crowded primary or secondary care centers with high workload and limited access to latest findings in the field.
MethodsThe expert-panel consisted of three pediatric gastroenterology and five pediatric allergy and immunology specialists from high-volume tertiary care hospitals based in Turkey. A literature search of PubMed/MEDLINE was conducted using the keywords [cow* milk allergy] and [infant] setting the publication date range from January 2010 to August 2018. In addition, Turkish publication database was searched using the same keywords to access all relevant articles locally published. Articles of interest were selected based on relevancy and experience. Current practices, international guidelines, and other relevant literature were discussed to form a series of recommendations aimed at pediatricians in Turkey, considering the challenges they face in daily practice, particularly in high-volume patient settings. Country-specific reimbursement policies and availability of replacement infant formulas were deliberated during the discussions.
EpidemiologyCMPA was found to have an incidence of 2–3% based on strict diagnostic criteria, although incidences of up to 7.5% have been reported in the literature.12,13 Unfortunately, there are no nationwide epidemiologic studies on food-allergy available in Turkey. A regional birth cohort study of 1377 infants in southern-Turkey (ADAPAR) reported the incidence of food challenge proven CMPA as 1.45%,14 and an earlier birth cohort study from the same region found the incidence of CMPA as 1.55% approximately 20 years ago.15 CMP is regarded as the most common food allergen for infants and young children. Preliminary analysis of a Turkish national multicenter study of children diagnosed with food-allergy (n=332; mean age 2.3 years) indicated CMP as the most common allergen (58.4%), followed by egg white (56.0%), and egg yolk (30.7%).16
Food-allergy prevalence in children was investigated in two surveys from the Black Sea region. In kindergarten and school-age children, parent-reported prevalence of food-allergy was 7.1% and 5.7%, while challenge proven prevalence of IgE-mediated food-allergy was 0.8% in both studies.17,18 Owing to the older age of children, CMPA was less pronounced in these studies with eggs, chocolate and beef-meat allergies reported as the predominant food-allergies.
Large-scale, comprehensive epidemiologic studies are urgently needed to determine the presentation, natural course, and future trends of CMPA in Turkey.
PrognosisCMPA has a favorable prognosis in most cases, resolving in 84–87% of the children by the age of three.13 Five-year follow-up of children with challenge-proven food-allergy in ADAPAR study showed that 80% of children with CMPA (16 of 20 children) developed complete tolerance by 5 years of age.19 EuroPrevall birth cohort study involving 9 European-countries and more than 9000 infants showed even earlier resolution of CMPA, with 69% of children developing tolerance by the age of two.20 Whereas another observational cohort from five referral centers in the United States found that only about half of the patients with CMPA developed tolerance by the age of five.21 Tolerance develops faster in cases with non-IgE-mediated CMPA, while strong and persistent CMP-specific IgE response, asthma, allergic rhinitis, urticaria, anaphylaxis and severe symptoms predict persistence of allergy.22 In a Turkish study investigating predictors of CMPA prognosis, high levels of specific IgE and STAT6 gene variant (GG genotype at rs324015) were determined to be indicative of delayed tolerance development.23
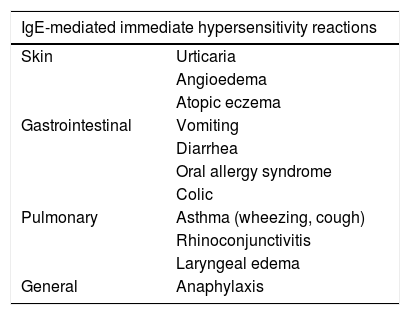
Clinical presentationCMPA may result in IgE-mediated immediate reactions (within minutes to 2h of exposure), non-IgE-mediated delayed reactions (hours or days after exposure), or a mix of both. Approximately half of CMPA cases are estimated to have IgE-mediated reactions.9 In a study of 54 children with suspected CMPA from Southern Turkey, 66.6% of patients were IgE positive.24 CMPA usually presents with ≥2 signs of variable severity and involves multiple systems. The most commonly affected organs are the skin (50–70%), the gastrointestinal system (50–60%) and the respiratory system (20–30%).13 Urticaria, angioedema, immediate vomiting, acute diarrhea, and asthma are usually associated with IgE-mediated CMPA (Table 1).
Signs and symptoms of cow’s milk protein allergy.
| IgE-mediated immediate hypersensitivity reactions | |
|---|---|
| Skin | Urticaria |
| Angioedema | |
| Atopic eczema | |
| Gastrointestinal | Vomiting |
| Diarrhea | |
| Oral allergy syndrome | |
| Colic | |
| Pulmonary | Asthma (wheezing, cough) |
| Rhinoconjunctivitis | |
| Laryngeal edema | |
| General | Anaphylaxis |
| Non-IgE-mediated or mixed type delayed hypersensitivity reactions | |
|---|---|
| Skin | Atopic eczema |
| Gastrointestinal | Gastroesophageal reflux |
| Enterocolitis | |
| Chronic diarrhea (± protein/blood loss) | |
| Constipation | |
| Iron deficiency anemia caused by occult blood loss | |
| Food-protein-induced enteropathy | |
| Food-protein-induced enterocolitis syndrome (FPIES – profuse vomiting, lethargy, pallor, diarrhea) | |
| Food-protein-induced proctocolitis (mild diarrhea and rectal bleeding; normal growth) | |
| Eosinophilic esophagitis (feeding difficulties, vomiting/gastroesophageal reflux disease, abdominal pain, dysphagia, food impaction) | |
| Pulmonary | Heiner syndrome (pulmonary hemosiderosis) |
| General | Failure to thrive |
Anaphylactic shock is a rare but life-threatening IgE-mediated reaction that may occur in up to 9% of children with CMPA.25 Milk allergy is responsible for 8–15% of fatal or near-fatal anaphylaxis cases.26 In a study of 45 hospitals in Istanbul province of Turkey, 12.7% of the anaphylaxis cases were seen in children younger than 10 years of age.27 A retrospective review of pediatric outpatient clinic records in a Turkish institution between 2010 and 2012 found 25 cases of food-induced anaphylaxis, most of which occurred in 0- to 3-year-old children; cow’s milk was the most common food allergen to cause anaphylaxis, accounting for 11 of 25 food-induced anaphylaxis cases.28 Though anaphylaxis criteria are defined adequately, rate of recognition and diagnosis by general pediatricians does not seem so high, leading to poor management and raising the rate of morbidity and mortality of children at risk.29,30
Gastrointestinal manifestations such as food-protein induced enterocolitis syndrome (FPIES), food-protein induced allergic proctocolitis (FPIAP), and food-protein induced enteropathy are suggestive of non-IgE-mediated CMPA. FPIES is diagnosed based on recurrent vomiting, lethargy, pallor, and diarrhea that improve with strict elimination diet, and absence of other IgE-mediated symptoms.31 FPIES generally presents when an infant is first exposed to cow’s milk or soy protein formulas or when solid weaning food are first introduced.32 In a large prospective cohort of 13,000 infants the cumulative incidence of CMP-induced FPIES was 0.34%.33 CMP-induced enteropathy, defined as a malabsorption syndrome in the jejunum similar to celiac disease, was mostly seen in 1960’s, but has declined to become a rare occurrence possibly due to increasing popularity of exclusive breastfeeding and improvements in infant formula compositions.34 In our literature search, we encountered one case of CMP-induced enteropathy from Turkey, in which a newborn with chronic diarrhea and failure to thrive was diagnosed with enteropathy based on duodenal biopsy revealing total villous atrophy and granulomatous duodenitis.35 The authors stated that symptoms resolved and normal growth ensued following 2-months of elimination diet with an amino acid formula.35 Eosinophilic esophagitis (EoE) and eosinophilic gastroenteritis can be found in mixed type CMPA showing characteristics of both IgE- and non-IgE-mediated immune reactions. Biopsy-proven EoE was determined to have a higher prevalence among patients with IgE-mediated food-allergy (4.7%).36 EoE has gained recognition as an allergic disease of the gastrointestinal system relatively recently. A pediatric case series published from Turkey followed 7 children diagnosed with EoE; 71% of children presented with food-allergy and 71% had comorbid allergic diseases, mainly asthma.37 Clinical response was achieved in all patients with elimination diet and/or inhaled corticosteroid treatment. As opposed to EoE, food-induced eosinophilic colitis is a benign disease seen as rectal bleeding in otherwise healthy breastfed infants, 2–3 months of age.38 Cow’s milk is the most likely allergen and fast recovery is seen with elimination of CMP-containing food from the mother’s diet.39
CMPA may often coexist with other types of food-allergies. In a survey of 38,480 children in the US, food-allergy prevalence was 8%; 38% of children with food-allergies had a history of severe reactions and 30.4% had multiple food-allergies. Among infants ≤2 years, CMPA prevalence was 2.90%, and 31.5% of them had multiple food-allergies.40 In Turkish studies 30% to 50% of OFC-proven patients had allergy to more than one type of food.18,24,41,42 Patients with CMPA or other food-allergies are also at increased risk of developing other atopic diseases such as atopic dermatitis, asthma, and rhinoconjunctivitis.43–45 CMPA and concomitant asthma were predictors of worse QoL scores in a recent Turkish study investigating quality of life in children with food-allergies.46 Family history of atopic disease is also a common finding. In reports from Turkey family history of atopic disease ranged from 28% to 52%.41,42,47
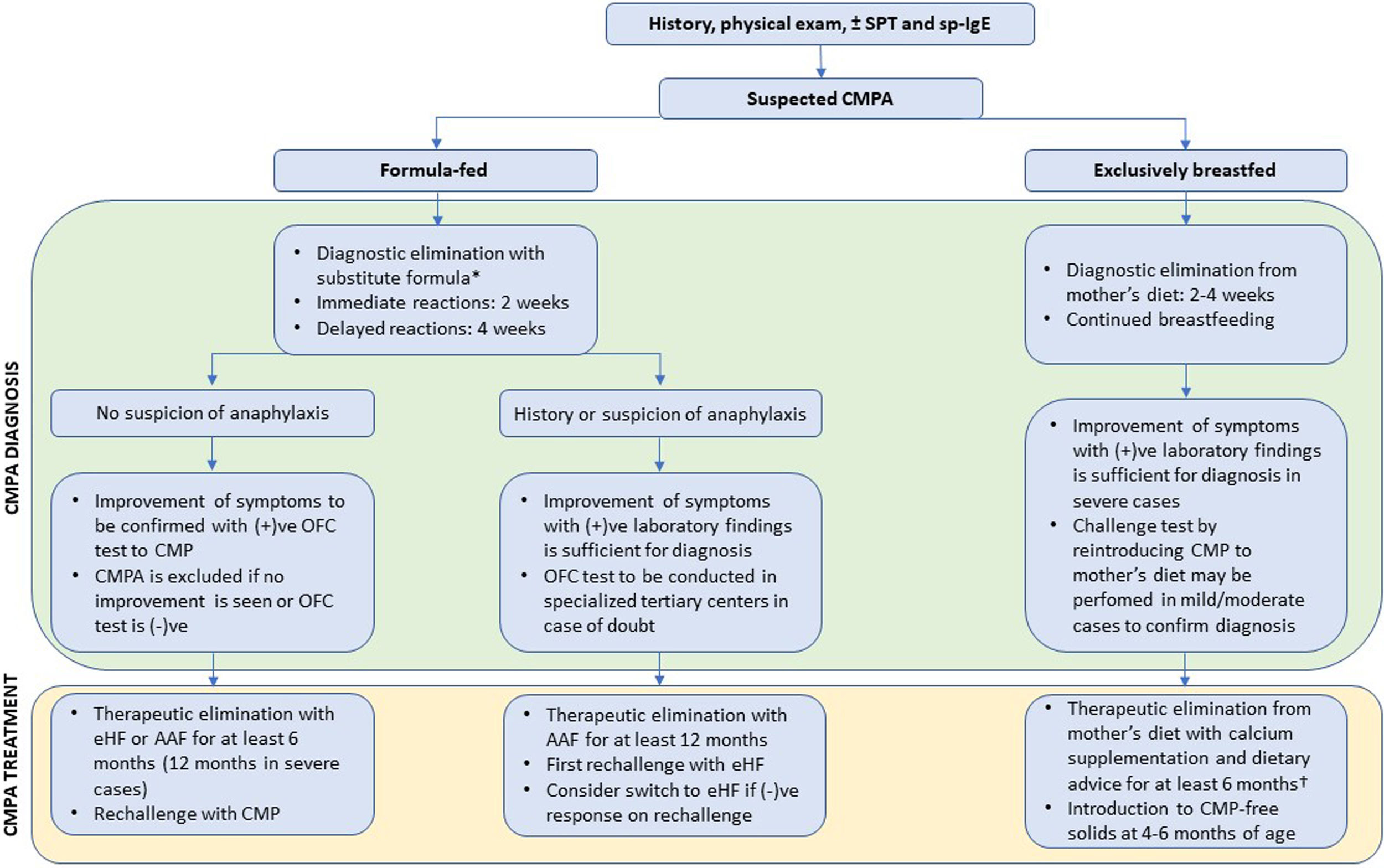
DiagnosisDue to the variety of signs and symptoms as well as variable symptom severity, diagnosis of CMPA is often challenging. Fig. 1 presents a proposed algorithm for diagnosis and management of CMPA.
Proposed algorithm for diagnosis and management of CMPA in infants. * To shorten the diagnostic process and minimize the risks of potential allergen exposure, we recommend AAF in the diagnostic elimination phase. CMPA can be definitively excluded if no improvement is seen with AAF, while it cannot be ruled out on eHF. AAF is strongly recommended in severe cases and those with any suspicion of anaphylaxis (e.g., contemporaneous findings of hypotension, bronchospasm, and angioedema). † CMPA symptoms in response to small quantities of CMP in breastmilk may be indicative of a severe reaction to CMP. If formula supplementation is needed in previously exclusively breastfed infants, AAF should be preferred as a precaution. Abbreviations: SPT, skin prick test; sp-IgE, specific IgE in serum; CMPA, cow’s milk protein allergy; (+)ve, positive; (−)ve, negative; OFC, oral food challenge; AAF, amino acid formula; eHF, extensively hydrolyzed formula.
To prevent unnecessary burden on patients and families and expedite the diagnostic process, it is important to get detailed patient history and perform complete physical examination. Skin prick test (SPT), and specific IgE testing in serum may support the diagnosis in cases of immediate reactions that are likely to be mediated by IgE (immediate vomiting, angioedema, urticaria, wheezing). These allergy tests have a high sensitivity but low specificity and should not be used as a diagnostic or screening tool in the absence of supporting clinical findings.48 The BSACI guideline suggests that in patients with typical CMPA history, a SPT wheal diameter of ≥3mm and serum specific IgE level of 0.35kU/L may be used as cutoff levels to determine test positivity.8 A recent systematic review investigated whether higher cutoffs could be used to correctly predict a positive OFC and concluded that in children <2 years old, CMPA diagnosis is highly likely if serum specific IgE level is ≥5kU/L or SPT with commercial extract yields a wheal diameter ≥6mm, but no clear cutoff level could be established for older children.49 As a general rule, all tests on human subjects should be performed in adequate clinical setting with trained personnel. Even a relatively noninvasive test as SPT has been shown to trigger anaphylaxis on rare occasions.50
EliminationIn case of suspected CMPA, the most reliable diagnostic method is to eliminate all CMP-based food from the diet and perform open oral food challenge (OFC) after improvement of symptoms to confirm the diagnosis.7,8,25 In case of suspected CMPA in exclusively breastfed infants, all cow’s milk products are eliminated from the mother’s diet for 2–4 weeks and breastfeeding is continued. It is also important to avoid other potential allergens to prevent confusion during the diagnostic phase. Improvement of symptoms can be seen within a week of elimination of all CMP-containing products for immediate reactions, but up to 4 weeks of elimination may be required to observe regression of delayed gastrointestinal symptoms.
Oral food challengeDiagnosis of CMP can be confirmed by an OFC test showing the return of symptoms. Although double-blind placebo-controlled food challenge (DBPCFC) is often cited as the golden standard, a positive open OFC test is considered as sufficient proof of CMPA in the majority of cases. DBPCFC is a difficult procedure and should be reserved for inconclusive cases referred to tertiary centers for further evaluation. As a fundamental principle, we recommend caution when implementing the OFC. The decision to perform OFC should be made individually based on patient’s history. The potential risks of anaphylaxis or other severe symptoms upon exposure should be weighed against the burden of unnecessary food avoidance. Laboratory tests may be sufficient for diagnosis in patients suspected of having severe IgE-mediated symptoms. OFC tests should always be conducted in the controlled environment of a fully equipped hospital, under the supervision of adequately trained medical staff. A study of OFC reactions in 122 preschool children with food-allergy in Turkey showed that OFC resulted in mild (19.0%) and moderate (4.5%) reactions; authors concluded that frequent testing with OFC is safe, especially when performed in patients with SPT wheal size and specific IgE concentration below the cutoff point.51
Formula substitutionChildren under the age of 2, who are not exclusively breastfed, require a substitute-formula to meet the age-appropriate nutritional requirements. Type of clinical manifestations, symptom severity, availability, cost-effectiveness and palatability should be considered when selecting the appropriate substitute-formula for each patient. Extensively hydrolyzed CMP-based formula (eHF;), hydrolyzed rice formula, soy-based formula, and amino acid formula (AAF) may be used as substitutes in children with CMPA. The two main types of substitute-formula widely available in Turkey are eHF and AAF. eHF is a hypoallergenic formula produced by extensive hydrolyzation of casein or whey proteins followed by hyperfiltration so that the molecular weight of peptides are <5000Da with majority <1000Da. The definition of hypoallergenic formula requires that it undergoes vigorous testing by DBPCFC trials to make sure that it is tolerated by at least 90% of children with CMPA.7 Conversely, AAF consisting of free amino acids in the place of protein is an allergen-free formula, since it contains no peptides that can elicit immune reactions.
Although eHF and AAF are considered equivalent in all nutritional aspects, international guidelines recommend the use of eHF as the first-line formula in uncomplicated cases.7–9 DRACMA and ESPGHAN guidelines reserve AAF for extremely severe and life-threatening cases such as anaphylaxis and eosinophilic esophagitis,7–9 while BSACI guideline gives a more detailed explanation for intended use of AAF, recommending it in patients with multiple food-allergies, severe CMPA, severe forms of non-IgE-mediated disease such as eosinophilic esophagitis, FPIES, enteropathies, failure to thrive, eHF intolerance, and in patients who developed allergic symptoms or severe atopic eczema when exclusively breastfed.8 We agree with the indications for AAF use mentioned in BSCAI, except for the latter: in Turkey, exclusive breastfeeding is recommended for the first 6-months, with elimination of CMP from the mother’s diet.
Studies suggest that 2–10% of infants with uncomplicated CMPA, and up to 40% of infants with complicated CMPA are intolerant to eHF, probably due to remaining CMP epitopes in the formula.52 eHF intolerance may lead to delayed diagnosis, persistence of symptoms, and additional visits to specialists. In patients who do not improve on eHF diet, switch to AAF successfully manages the allergy symptoms and provides adequate nutrition, restoring the growth parameters.53 Anaphylaxis is a major concern, especially for first-time users of formula, who may not have a prior history of anaphylaxis. Based on very low quality of evidence, DRACMA guideline defined high-risk of anaphylaxis as having a prior episode of anaphylaxis and currently not using eHF, while low risk of anaphylaxis as no prior anaphylaxis episodes and currently being on eHF.9,25 However, these definitions do not account for sensitization that develops over time to induce anaphylactic reaction. A single-center retrospective study of CMPA patients between 2005 and 2008 revealed 6 cases of severe systemic reaction upon exposure to eHF, suggesting that no hydrolysate can be considered 100% safe.54 A more recent report also revealed two cases of immediate systemic reaction to eHF and used ELISA inhibition tests to show that two of three whey-based eHF brands contained whey and casein peptides that were recognized by specific IgEs in patients’ serum.55 It is the opinion of this expert-panel that anaphylaxis risk may not be classified as high or low based on current evidence. We recommend that the physicians should evaluate any risk of anaphylaxis as real and err on the side of caution.
The main reason for recommending eHF as the first-line formula is the higher costs of AAF in most countries, which according to a recent review may be 6–8 times more expensive than eHF.6 However, depending on country-specific formula costs some studies have found similar or better cost-effectiveness for AAF. A recent Brazilian pharmacoeconomic study considering the eHF intolerance has shown that use of AAF in diagnostic elimination test of infants suspected of having CMPA was significantly more cost-effective and resulted in more symptom-free days than using eHF, even though AAF was twice as expensive as eHF.56 Similarly, a study from Australia found that initial prescription of AAF to all CMPA cases would result in similar healthcare costs as prescribing according to current guidelines, even though AAF was three-times more expensive than eHF; a higher number of doctor visits and interventions were required in patients who could not tolerate eHF accounting for similar overall costs under both scenarios.57 Thus, considering the favorable reimbursement system in Turkey where AAF is only 25% more expensive than eHF, AAF can be used as a first-line substitute, particularly during the period of diagnostic elimination. A cost-minimalization model from Turkey supports this view: according to monetary values valid on March 2018, cost of treating a child with CMPA for two years was estimated as 9505TL with AAF and 9582TL with eHF.58
Long-term managementThe most effective method to prevent recurrence of symptoms is strict avoidance of all CMP-containing products. Milk from other mammals such as goat or donkey or partially hydrolyzed formulas containing higher molecular weight peptides are not suitable for CMPA patients, as cross-reactivity between species and presence of allergens may elicit immune reactions. In a Turkish study of 24 children with challenge-proven IgE-mediated CMPA, 79% of children (19 of 24) showed sensitivity to goat’s milk based on prick test.59 The use of baked milk products is encouraged in tolerant patients as it widens the food choices and increases the quality of life for the patients and their families. However, those with no history of exposure would have to be tested for tolerance with an OFC in a controlled hospital environment before declaring it safe to consume. In a Turkish study of 57 infants with CMPA, following 6-months of elimination phase majority of children with either IgE- or non-IgE-mediated CMPA were tolerant to baked milk and two-thirds of non-IgE-mediated CMPA were tolerant to yoghurt.60
Milk constitutes an important part of regular infant diet and appropriate nutritional guidance should be provided to patients and families upon its elimination. Label-reading and looking out for hidden ingredients should be emphasized. Growth and development parameters should be monitored regularly and age-appropriate food substitutes should be provided to prevent vitamin and mineral deficiencies. Dietary counselling should be individualized according to age, variety and severity of symptoms, and coexisting allergies61 and assess and address any comorbid feeding difficulties. Complimentary solid foods can be started without delay at 6-months of age. There is no evidence to suggest any benefit in postponing other allergenic foods.
Development of toleranceSpontaneous tolerance develops in most patients before school age. Depending on symptom severity patients may be subjected to OFC every 6–12 months in a controlled hospital environment to assess tolerance. In severe cases, eHF or baked milk may be used for challenge before moving on to fresh pasteurized cow’s milk. OFC with baked milk may be preferred in patients expected to have a positive reaction to the test, as symptoms tend to be milder with baked milk than fresh milk.8
Tolerance to baked milk is regarded as to be a predictor of good prognosis, with most patients outgrowing CMPA within a few years.62 However, it is not clear whether baked milk consumption has a role in developing further tolerance to fresh milk; a recent systematic review found no evidence that baked products accelerate the development of milk or egg tolerance.63
A nonrandomized study investigating the effect of substitute-formula on development of tolerance in children with CMPA suggested that among five different milk substitutes, use of eHF+Lactobacillus rhamnosus GG (LGG) led to significantly higher rate of tolerance at the end of 12 -months.64 However, the results of this study were prone to bias since formula selection is dependent on various factors such as organ involvement, symptom severity, and test results, all of which ultimately affect the development of tolerance. The same group then conducted a randomized controlled trial with parallel arms receiving either eHF or eHF+LGG and determined that addition of LGG to eHF reduced the incidence of other allergic manifestations such as eczema, urticaria and resulted in faster development of tolerance.65 Another recent randomized controlled trial investigated gut microbiota in non-IgE-mediated CMPA infants fed AAF with or without synbiotics (a combination of prebiotic fructooligosaccharides and probiotic bifidobacterium Breve M-16V) and showed that AAF+synbiotic treatment successfully modified the composition of gut microbiota to the levels seen in healthy breastfed controls.66 However, the study was not designed or powered to demonstrate a clinical difference between the two treatment arms. While probiotic treatment appears promising, we feel that current evidence is insufficient to endorse their use in everyday practice.
Oral immunotherapy with initially very low, escalating doses of CMP has been a current hot topic. Results from a systematic review suggests that desensitization against food allergens can be achieved during treatment phase; however, lasting effectiveness of treatment post-discontinuation is not clear.67 The European Academy of Allergy and Clinical Immunology (EAACI) recommends that oral immunotherapy can be performed in research and clinical centers with extensive experience for children with persistent food-allergies, at least 4–5 years of age, harboring no contraindications to treatment.68 Given the significant risks and the experimental nature of oral immunotherapy, we do not recommend its use outside of research centers with extensive experience.
PreventionMeasures to prevent food-allergy or atopic disease, especially in high-risk infants, have been investigated in several studies. Children with moderate-severe eczema in early life or history of allergy or atopic disease in the immediate family are considered at high-risk of allergy development. Current data suggest that avoidance of allergen consumption during pregnancy or lactation by the mother does not provide any protective effect for infant in later life.69,70 Some studies now suggest that early introduction of solid foods at 4–6 months, including those with allergenic properties, along with continued breastfeeding may confer tolerance in high-risk infants.71 However, results of ongoing randomized studies are awaited for a final verdict on this matter. Some guidelines and reviews recommend extensively or partially hydrolyzed formulas to prevent food-allergy in high-risk infants.69,70 However, a recent systematic review challenged these recommendations, since the majority of evidence supporting their use were biased or flawed in design.72
We recommend exclusive breastfeeding for the first 6-months in all infants regardless of risk level and support the introduction of complementary solid foods without delay. Other preventive strategies require further evidence of success before country-wide application.
ConclusionThe higher workload of pediatricians and family physicians in crowded primary and secondary healthcare centers in Turkey makes adequate history taking and physical exam a challenge, although these are the cornerstones of CMPA diagnosis. Our purpose is to increase awareness and provide concise practical recommendations to help physicians recognize the symptoms of CMPA, leading to faster diagnosis and management relieving the burden of patients and families.
CMPA suspicion should be confirmed by elimination of CMP resulting in symptomatic improvement and OFC test showing a return of symptoms. Diagnostic elimination phase lasts 2–4 weeks and involves elimination of all CMP-containing food from mother’s diet in breastfed infants and switch to a hypoallergenic formula in formula-fed infants. OFC test should always be conducted under expert medical supervision. SPT and specific IgE measurement in serum may be used to support CMPA diagnosis in IgE-mediated allergy. Therapeutic elimination with appropriate diet modification should last at least 6–12 months before tolerance development is tested by rechallenge in clinical setting.
AAF and eHF are the two main CMP substitutes in Turkey. AAF is a non-allergenic formula recommended for patients with presence or risk of anaphylaxis and other severe symptoms, particularly multiple food-allergies, eosinophilic esophagitis, food-protein-induced enterocolitis, food-protein-induced enteropathy, failure to thrive, and as supplement in previously exclusively breastfed infants with CMPA. In addition, AAF is recommended for patients who cannot tolerate eHF, which occurs in 2–10% of patients with noncomplicated disease and up to 40% of patients with complicated disease. AAF may also expedite the diagnosis, since lack of improvement definitively excludes CMPA, while infants showing lack of improvement on eHF diet need further testing with AAF before CMPA can be excluded. Although international guidelines limit the use of AAF to cases of absolute necessity, citing AAF as 6–8 times more expensive than eHF, the price difference between these two formulas is approximately between 25% to none in Turkey.
CMPA encompasses a wide spectrum of signs and symptoms of variable severity. CMPA management should be individualized according to the clinical context such as severity of disease, risk of anaphylaxis, and tolerability, as well as social context and access to medical services.
Declaration of interestThe authors have no conflict of interest to declare.
FundingNutricia Turkey provided unconditional research grant.
We thank Anil Aksit and Feza Kirbiyik from Nutricia Turkey for unconditional research grant and Mehmet Berktas, MD MSc MICR from Blue Idea Consulting - United Kingdom and Beril Tavsanli Sili, PhD for their editorial support.