INTRODUCTION
As early as 1912 various proteins of egg white were implicated in egg allergy by both in vivo and in vitro investigations 1. The first report examined a child with acute egg hypersensitivity in which egg-white and ovomucoid, but not egg yolk, were reported to be active allergens by food challenge. Ovoglobulin and ovomucin were found to have only 25 % of the activity of ovomucoid 1. Miller and Campbell 2, on the other hand, reported that individuals sensitive to egg white reacted to ovomucoid, ovomucin, ovalbumin and lysozyme by skin test. There was no overall pattern of reaction and they suggested that sensitisation to egg white allergens is dependent upon individual variation rather than the nature of the antigen. Ovomucoid was reported to be the major skin-reactive protein in both raw and cooked egg white by Bleumink and Young 3. They observed no reaction to ovalbumin, ovotransferrin and ovoglobulin. However, they did observe a reaction to lysozyme which they attributed to its irritant properties. Earlier investigations of raw and cooked egg are in conflict with this report. Ratner et al 4 with the aid of passive transfer tests found that ovomucoid-sensitive individuals reacted to hard-boiled eggs while patients allergic to ovalbumin and ovoglobulin tolerated hard-boiled eggs. Conversely, Rhoden and Sutherland 5 found that patients allergic to raw egg white reacted strongly to ovomucoid, while those sensitive to the heat labile portion of egg white also reacted with ovomucoid.
With the advent of improved immunological assays such as the radioallergosorbent test (RAST) the ability to define which proteins were involved in IgE-mediated egg allergy was greatly improved. Hoffman 6 reported that 25 out of 27 patients displaying symptoms on ingestion of egg gave positive RAST results. Three patients with strong anaphylactoid reactions to egg showed positive RAST tests to ovalbumin and ovomucoid. Both allergens may, however, have been slightly cross-contaminated. A later study by the same author 7 using higher purity egg proteins indicated that ovalbumin, ovomucoid and ovotransferrin were important allergens, whereas lysozyme was only a weak allergen. Ovalbumin and ovomucoid and small amounts of ovotransferrin were detected in both hard- and soft-boiled eggs by radioimmunoelectrophoresis.
At this time Langeland 8 and Langeland and Harbitz 9 reported that for a group of egg-allergic children the most important allergens detected by crossed-radioimmunoelectrophoresis were ovomucoid, ovalbumin and ovotransferrin. Lysozyme was not detected as an allergen in this way. However, Holen and Elsayed 10 found that lysozyme bound strongly to IgE in all of the sera of egg-allergic individuals that they studied, and concluded that lysozyme was one of the major allergens of egg white.
Egg yolk has largely been ignored by researchers, as early reports indicated that it was not allergenic 11. However Anet et al 12 recorded positive RAST scores for egg yolk in the sera of 36 egg-allergic patients. They also found that lysozyme bound specific IgE in the sera of four egg-sensitive patients. We have also recently reported IgE binding to several yolk proteins 13, 14. Some egg allergen fractions may be expected to give a high incidence of false positives in vivo due to their irritant property (e.g., lysozyme), especially for patients with atopic eczema. Often test procedures and materials were not well defined in the early literature. However these last two factors may not account for the wealth of conflicting reports of allergenicity. Therefore we have investigated binding of specific IgE in the sera of 40 egg-sensitive children to 8 purified egg white and yolk proteins by RAST in an effort to determine which of them are major allergens. The proteins were purified by high performance liquid chromatography (HPLC) where necessary to ensure purity. The results appear to explain the conflicting nature of reports on the allergenicity of egg proteins.
MATERIALS AND METHODS
Sera of egg-allergic patients
Sera were obtained from patients who were referred to the Allergy and Clinical Immunology Unit of the Royal Childrens Hospital, Melbourne, for evaluation of suspected hypersensitivity reactions to egg. At the first assessment a detailed clinical history was taken. Where the reaction was not life-theatening and of doubtful significance, or had occurred more than 12 months prior to referral, a home challenge of soft-boiled egg was undertaken to confirm clinical evidence of egg hypersensitivity. Where there was unequivocal clinical evidence of persisting egg hypersensitivity, patients attended the Allergy and Clinical Immunology Unit. Here skin tests to common allergens (including egg) were performed and blood samples taken for estimation of total immunoglobulin levels and allergen-specific IgE antibodies. Patients used in this study were selected for both clinical sensitivity to egg and a 3 + or 4 + Phadebas RAST score to Pharmacia egg white discs.
Allergens
Ovalbumin was prepared by three times recrystallisation of egg white from an ammonium sulphate solution. The solution of impure ovalbumin was then chromatographed on a DEAE-Sephacel column equilibrated with 0.05 M Tris-HCl buffer (pH 8.0) containing 0.05 M sodium chloride. Ovalbumin was eluted from the column using a linear gradient of 0 to 1 M sodium chloride in 0.05 M Tris-HCl, pH 8.0. Lysozyme was prepared by the method of Alderton and Fevold 15. The crystals were then further purified by chromatography on a DEAE-Sephacel column equilibrated with 0.05 M Tris-HCl buffer (pH 8.0) containing 0.05 M sodium chloride. Lysozyme was eluted from the column by a linear gradient of 0 to 2 M sodium chloride in 0.05 M Tris-HCl, pH 8.0.
Ovotransferrin (98 % pure) was purchased from Sigma Chemical Co, Missouri, USA. It was purified by ion exchange HPLC on a Brownlee X03-MP aquapore cation column.The sample, dissolved in 0.015 M NH4HCO3 (pH 4.7), was injected and then eluted with the same buffer until all unbound material was removed. The ovotransferrin was then eluted with a linear gradient of 0.015 (pH 4.7) to 0.35 M (pH 6.5) NH4HCO3. The material thus eluted was finally purified by HPLC on a Waters Associates reversed phase C18 microbondapak column using a 30 to 70 % linear gradient of acetonitrile in water. Ovomucoid was prepared according to the method of Kurisaki et al 16.
Ovomucin was prepared by the procedure of Kato et al 17. Homogenised thick egg white (150 mL) was centrifuged at 59000 g for 60 minutes to separate thick white into a supernatant and a gel-like precipitate. The supernatant, containing disaggregated ovomucin, was diluted fourfold with 0.05 M barbital buffer, pH 8.6, and applied to a Sepharose 4B column. Fractions were eluted with the diluent buffer. The fraction which appeared in the void volume was denoted disaggregated ovomucin. This preparation was shown to be slightly contaminated with ovalbumin by gel electrophoresis. Ovalbumin was removed by rechromatography in the same fashion. Gel electrophoresis 18 of the void volume fractions revealed 4 bands of high molecular mass (Mr > 94000) corresponding to ovomucin containing various amounts of carbohydrate. These were shown elect rophoretically not to be contaminated with low molecular mass proteins including ovotransferrin, ovalbumin, ovomucoid or lysozyme.
Apovitellenins I and VI were prepared according to the methods of Burley and Davies 19 and Burley and Sleigh 20, respectively. Phosvitin was isolated by a modification of the procedure of Wallace and Morgan 21 in which the enzyme inhibitor was omitted and salt was removed by dialysis against distilled water. The purity of each of the above allergens was determined by SDS gel electrophoresis on a 10 % T gel 18. Every allergen gave a single band in this system except ovomucin, which has multiple glycosylated forms and appeared as several bands. The ovomucin preparation was shown to be free from contamination by other proteins 14.
Preparation of RAST discs and RAST procedure
Filter paper discs (6 mm) were activated with cyanogens bromide by the methods of Ceska et al 22. Since apovitellenin I and ovomucin are poorly soluble in the NH4HCO3 buffer used for coupling of allergens to discs, any experiments using these proteins were done utilising nitrocellulose as the solid phase 23. The optimal amount of each protein to be coupled was determined by constructing a binding curve in which increasing amounts of the allergen was added to CNBr-activated paper or nitrocellulose discs 13,14. RAST was then performed using these discs and one serum sample with high levels of IgE specific to the particular protein. Exposure to a standard amount of 50 µg of allergen/disc was employed for each of the allergens. The RAST procedure followed the manufacturer's instructions (Kallestad, USA).
Skin tests
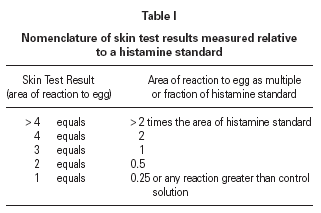
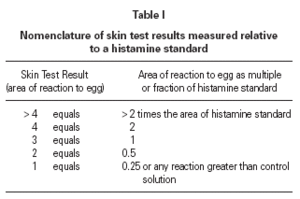
The size of a test weal produced by an egg white allergen extract (Dome Hollister Steir, Spokane, USA, 1:20 w/v) was compared with that from histamine acid phosphate (1 mg/mL) and from a control. Skin prick tests were performed by a single investigator by placing 1 drop of the extract, control solution and histamine on the patient's back. The point of a blood lance needle was passed through the drop to indent the skin but not to cause bleeding. The size of the skin weal elicited by the egg white extract was compared with that those of the histamine and the control solutions. The area of weal and pseudopodia elicited by the histamine was designated as + 3. Reactions to egg were graded as shown in table I. The weal produced in skin prick test of the manufacturer's control solution was deducted from that of the egg allergen before the score test was measured. Skin tests were carried out at the same time as blood was taken for immunological studies.
Statistical analysis
The RAST results were tabulated for the 40 patients in a random order (achieved by testing the sera in the sequence that they were received at the clinic). Since no analysis has been done in the past to establish any pattern of allergenicity for each individual protein, a correspondence analysis (Greenacre 24) was performed in order to explore the possibility of any interrelationships. Correspondence analysis is a graphical technique for simultaneously representing the rows and columns of a data matrix in such a way as to identify the structural relationships between the results in the rows and columns. To analyse the data it was tabulated into rows representing the 40 children and columns representing the percent radioactive uptake in RAST to the 8 proteins.
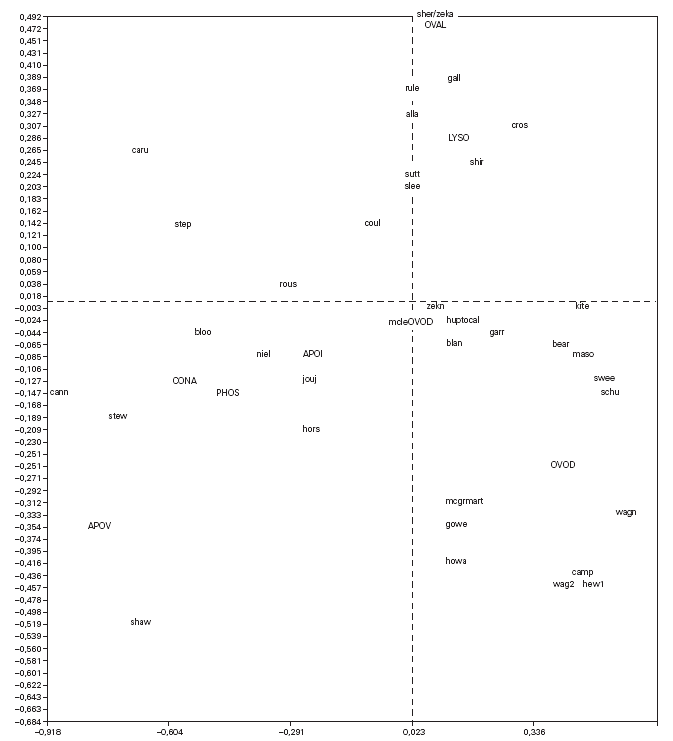
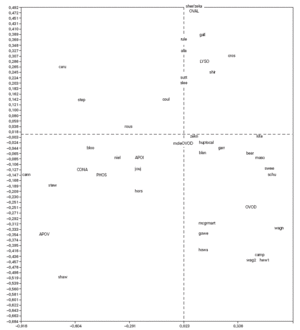
Correspondence analysis was carried out and showed the data as two sets of points (fig. 1), one representing the patients (rows) and the other standing for the proteins (columns), on a joint map. Each point corresponding to a patient can be considered a display of the complete reactivity profile for that patient. The information has been condensed into a single point. Furthermore, the distance between the points for the patients is a measure of similarity between the RAST scores for their sera with the allergens. Thus in fig. 1, the points for the patients "caru" and "camp" are far from each other because their RAST scores for the 8 proteins are different, while the point for patient "sutt" is close to that of "slee" because their profiles are similar across the RAST scores for the 8 allergens.
Figure 1.--Correspondence analysis diagram showing each patient point and each allergen point as a four letter code. Abbreviations are as follows: OVAL ovalbumin, LYSO lysozyme, OVON ovomucin, OVOD ovomucoid, APOI apovitellenin I, APOV apovitellenin VI, PHOS phosvitin, and CONA ovotransferrin. See Materials and Methods for explanation of diagram.
The points depicting the allergens were interpreted in much the same way as correspondence analysis treats the rows and columns in a matrix. Thus each allergen point portrays the profile of RAST scores for that allergen across the set of 40 patients. So it is apparent from fig. 1 that the profile across the patients for apovitellenin VI is very different from that of lysozyme. The relative positions of the two sets of points are interpreted in a special way in correspondence analysis. Each patient point will lie more or less in the region of the allergen score in which the patient's profile is prominent. Consequently the point for child "shir" is near that of lysozyme because this patient has a relatively high RAST score for lysozyme while having comparatively low scores for the other proteins. In this way groupings of patients and allergens were determined.
RESULTS
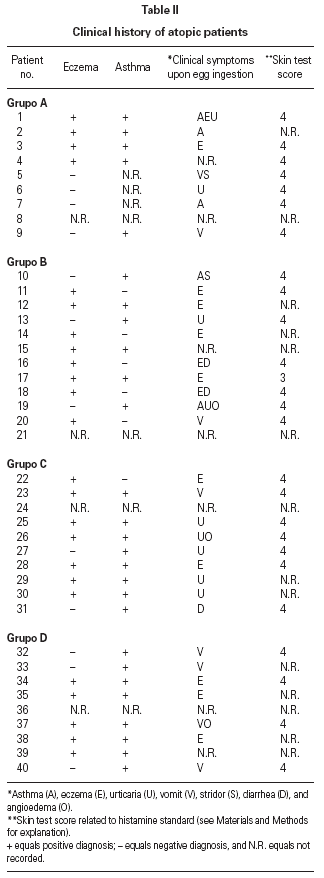
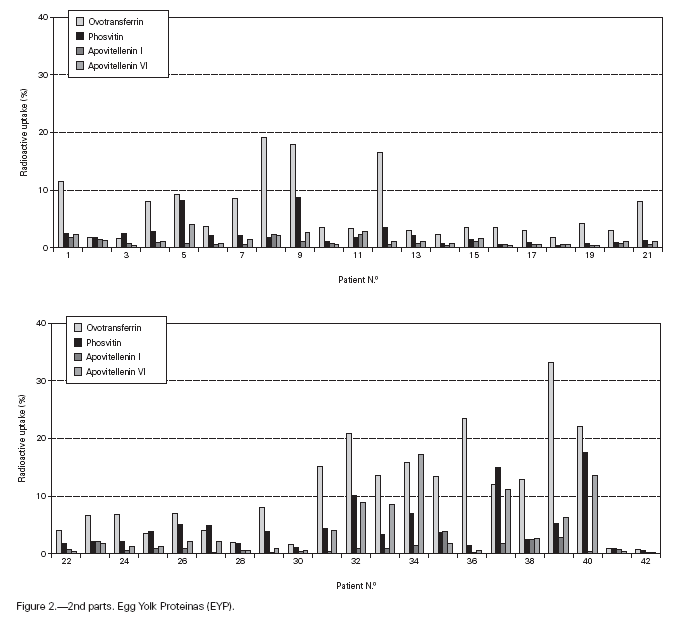
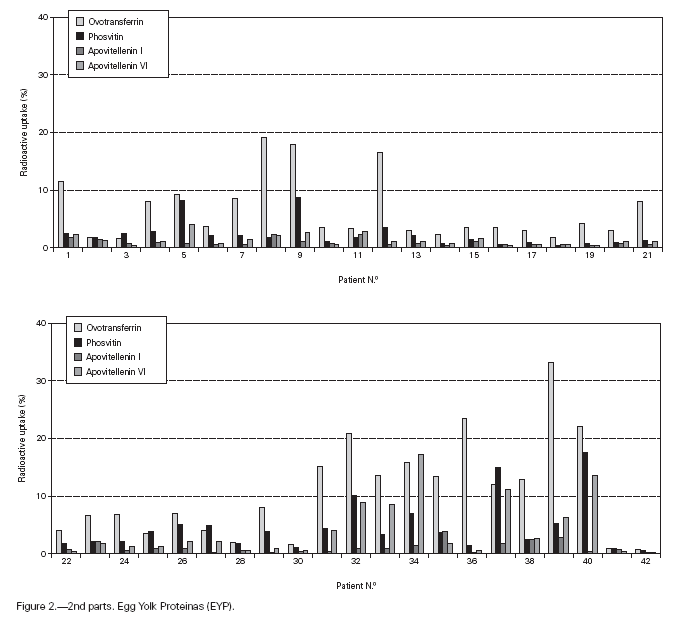
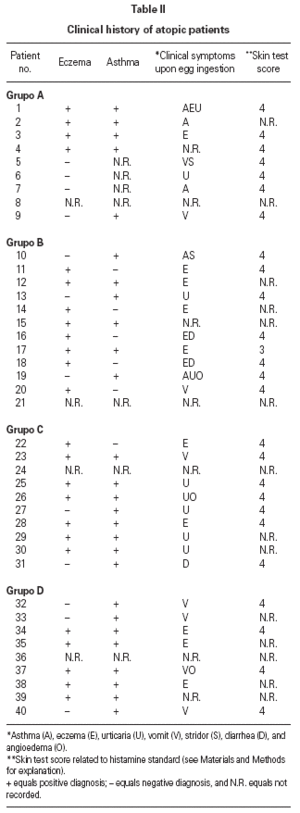
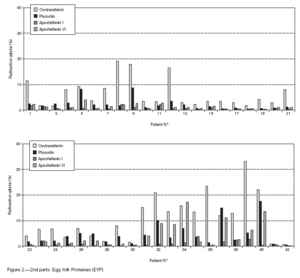
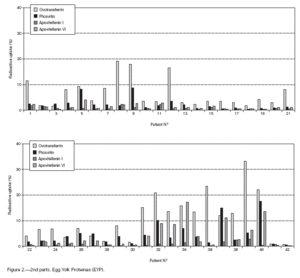
Table II gives some clinical details for the group of patients used in this study. The symptoms observed upon ingestion of egg varied, and there is no distinct pattern of reactivity amongst the groups listed. Patient numbers 41 and 42 correspond to foetal cord serum and a housedust mite allergic serum respectively, and were used as controls for non-specific binding. For ease of comparison with figure 1, table II has been arranged according to the groups defined by the correspondence analysis. Figure 2 shows for all of the patients the percent radioactive uptakes for the purified egg white and egg yolk proteins. The results have been ordered in the groups determined by the outcomes of the correspondence analysis.
Figure 2.--RAST results, shown as percent radioactive uptake, for the egg white and yolk proteins on discs reacting with IgE in the sera of 40 egg-sensitive children. Results have been drawn according to the groups in which they were placed by the correspondence analysis (see Materials and Methods). The results for two control sera are also shown. These sera were foetal cord serum (no. 41, low IgE) and a serum with high non-egg specific IgE (no. 42).
The correspondence analysis indicated, firstly, that the allergens were related in their IgE binding across the 40 patients according to the following sets: set 1, the egg white proteins (EWP) lysozyme and ovalbumin; set 2, ovomucoid (EWP); set 3, ovomucin (EWP); and set 4, ovotransferrin (EWP) and the egg yolk proteins (EYP) apovitellenins I and VI and phosvitin. Secondly, the analysis clearly divided the patients into four groups. Group A is comprised of patients numbers 1 to 9 whose sera reacted most similarly to ovalbumin and lysozyme (EWP). The sera of this group generally did not display IgE binding to Set 4 allergens. Group B consists of patients 10 to 22 and their sera reacted most similarly to ovomucoid (EWP). Their sera generally showed little or no IgE binding to any of the other allergens.
Group C is made up of patients 23 to 30. Their sera reacted most similarly to ovomucin (EWP). Their sera generally showed IgE binding to most of the egg white proteins, but the sera of all members of the group had higher radioactive uptakes for ovomucin than for the other sets of allergens. The sera of this group did not show significant IgE binding to any egg yolk proteins. Group D contains patients 31 to 40. Their sera reacted most similarly to the set 4 proteins. Their sera generally gave high IgE binding to ovalbumin and ovomucoid. Also the sera of all members of the group had notably high RAST scores with ovotransferrin. This group was the only one whose sera showed significant IgE binding to yolk allergens.
DISCUSSION
The results in figure 1 indicate that ovomucoid, ovalbumin and ovotransferrin are indeed important allergens for many individuals, at least by an in vitro test. This is in accord with earlier findings 3,7,8,9. However, our results clearly show that there are a number of other allergens important for various groups of patients. Both lysozyme and ovomucin bound significant amounts of IgE in the sera of patient groups A and C. Lysozyme was statistically in a set with ovalbumin and was a significant allergen for Group A. This is consistent with the findings of Miller and Campbell 2 and Anet et al 12, but in disagreement with the report of Langeland and Harbitz 9. Although the yolk allergens tested generally did not bind as much IgE as did the egg white proteins, IgE in the sera of Group D did react with the former. Interestingly, IgE in the sera of all members of this group also showed a relatively high level of binding to ovotransferrin. This agrees with the results of Langeland 8 who found that there was allergenic cross-reaction between ovotransferrin and whole egg yolk. Whether all three of the yolk proteins tested cross-react or only one or two of them is not revealed by our results.
The allergens that are grouped together by our results, that is, lysozyme and ovalbumin (set 1) and ovotransferrin, apovitellenins I and VI and phosvitin (Set 4) may have common allergenic determinants. Alternatively, a person may be genetically predisposed to produce IgE antibodies to one group of egg proteins or another but not to all of the groups. We are led to return to the suggestion of Miller and Campbell 2 that sensitization to egg allergens is dependent upon variation in patients but not upon the nature of the allergens. This would explain our results leading to identification of groups of patients clearly defined by their IgE specificity, although the clinical symptoms that are displayed by members of the groups were not significantly different from each other. It may be that some other, as yet unidentified, clinical indicator would help to differentiate between the groups. This possibility should be examined.
The existence of such patient groups may explain why various workers have reported different allergens to be important in egg hypersensitivity. For example, those examining only ovalbumin and ovomucoid would miss two groups of patients whose sera contain IgE directed against other egg proteins. Also a sufficiently large number of patients must be examined so as to provide a representative distribution across each group, otherwise the results may be biased towards one or more allergens. Finally it would appear that although some egg allergens are less stable to heat than others, in cooked egg they are still capable of generating significant levels of specific IgE.
ACKNOWLEDGEMENTS
This work was supported by the National Health and Medical Research Council of Australia. The authors thank Dr. R. Burley for the generous gift of purified apovitelleninsI and VI and of phosvitin.