the RhinAsthma Patient Perspective (RAPP) is the only available tool to assess HRQoL in daily practice. The aim of this study is to cross-culturally validate the RAPP in Spanish.
MethodsThe RAPP was translated into Spanish. Adult patients receiving usual care for asthma and allergic rhinitis (AR) were recruited consecutively and assessed twice with a four-week interval between visits to test the psychometric properties of the questionnaire.
Results149 patients (62.8% female) with a mean age of 37.7 years completed the study. Exploratory and confirmatory factor analysis confirmed the uni-dimensional structure of the questionnaire. Internal consistency (0.73 at visit 1; 0.87 at visit 2), convergent and discriminant validity (p<.05 at both visits) were satisfactory. Reliability was confirmed by an ICC of 0.69 and a CCC of 0.74. Responsiveness was supported by a significant association with VAS (r=0.28, p<0.003) and ACT (r=−0.35, p<0.01). The minimal clinical difference (MID) value in the analyzed population was 2.
ConclusionsThe Spanish version of RAPP was demonstrated to have satisfactory psychometric properties and is a valid, reliable and responsive tool for the assessment of asthma and AR HRQoL in clinical practice.
In the field of respiratory allergy, Health Related Quality of Life (HRQoL) has been implemented as a key outcome in clinical trials and observational studies, with the aim of comparing groups of individuals.1 A large number of questionnaires have been developed for clinical research purposes to assess the consequences of allergic rhinitis (AR) and asthma from the patient’s perspective.2,3 Although the integration of HRQoL measures into the workflow of routine care is being encouraged it has not been, to date, satisfactorily implemented in respiratory allergy. This depends mainly on the lack of validated tools for use with individual patients. When HRQoL is assessed as research outcomes, patients fill in the questionnaires and the collected data are analyzed in aggregate; the individual results are not usually seen by patients or their physicians, hence no individual actions are taken based on this information. In routine practice, the collection of HRQoL data has the aim of offering clinicians and patients a standardized approach to monitor the impact of the disease, assess treatment outcomes and allow patient’s engagement in shared decision-making and self-management.4 To be used in clinical practice, HRQoL tools must be short and simple to complete, score and interpret. Moreover, they must be validated to be applied to individual patients and satisfy the defined standards of validity, reliability and responsiveness.5
The RhinAsthma patient perspective [RAPP]16 is a questionnaire of eight items for routine use to assess the HRQoL of patients with asthma and comorbid allergic rhinitis (AR). Patients indicate the extent to which they were bothered by each problem during the two weeks preceding the completion of the questionnaire on a five-point Likert scale (not at all, a little, fairly, much, very much). The score is a simple sum of the single answers (range 8–40), with higher scores corresponding to a worse HRQoL. A cutoff point of 15 demonstrated the best sensitivity and specificity in discriminating the achievement of an optimal HRQoL.6
The questionnaire possesses all the characteristics that are required for use with individual patients. The RAPP has been validated in Italian and it is now also available in Portuguese.7 To date, it remains the only available tool that can be used in clinical settings to monitor HRQoL of patients with respiratory allergy. To be used in languages different from the original one, questionnaires must be fully cross-culturally adapted and tested to verify the psychometric characteristics, following well-established procedures.8 The need to assess the individual perspective in clinical practice and the importance that the current guidelines attribute to HRQoL,9 encouraged us to validate the RAPP into Spanish, thus providing the medical profession for Spanish and other Spanish-speaking patients with a proper tool to evaluate the impact of disease and treatment on their life.
Spanish, an official language in 21 countries, is the second most commonly spoken language in the world.10,11
The aim of this study was to translate and cross-culturally adapt the RAPP into Spanish and to evaluate the psychometric properties of this adapted version following the available guidelines.12,13
Materials and methodsIn line with the international guidelines for cross-cultural adaptation, two forward and backward translations were performed.14 Once the Spanish version of RAPP was obtained, patients who had a scheduled visit at the Department of Allergy and Immunology Hospital Quironsalud Bizkaia, Bilbao, were enrolled between February 2017 and May 2018. Patients were selected using a convenience sampling method.15 The aim was to include 150 patients (age ≥18), with a diagnosis of asthma and AR according to GIN 16 and ARIA17 guidelines, and who were willing to participate. Patients suffering from other respiratory or ear–nose–throat disorders were excluded.
This study was part of a multinational project aimed at validating the RAPP in English, French, Portuguese, Spanish and Polish.
The study protocol was approved by the University of Genoa Ethics Committee (approval no. P.R. 333REG2016) and ratified by the local ethics committee. It complies with the general principles of Good Clinical Practices and the Declaration of Helsinki as amended in Edinburgh in 2000. Only patients who provided written informed consent were included in the study.
Enrolled patients were scheduled for a follow-up visit four weeks later. At the first visit, sociodemographic and anthropometric data, disease patterns, spirometric values, smoking habits, and current treatment were collected.
At each visit patients filled in the RAPP questionnaire along with the following tools:
- -
Short Form Survey (SF-12)18: the short form of the 36-item Short Form Survey [SF-36],19 is a validated tool for measuring health status. It consists of 12 items and provides two separate summary scores: a physical component score (PCS) and a mental component score (MCS). The sum score ranges from 0 (the worst possible health) to 100 [the best possible health].
- -
Asthma control Test (ACT): this is a validated tool to assess the level of asthma control.20 It consists of five items that evaluate limitations at work, school and home because of asthma, the amount of dyspnoea, the presence of nocturnal symptoms, the use of rescue medications and the patient’s subjective perception of their level of asthma control over the previous four weeks. Patients assign a score from 1 [poorest control] to 5 [total control] to each item and the sum of them identifies three levels of control: uncontrolled asthma [scores from 5 to 19]; well-controlled asthma [scores from 20 to 24]; and totally controlled asthma [score of 25].
- -
Symptomatologic Visual Analog Scale (VAS)21: this simple quantitative measure was proposed by the Joint Task Force on Practice Parameters to assess AR symptom severity.22 Patients are asked to indicate the global discomfort caused by their AR during the previous week using a 100-mm long horizontal line with verbal descriptors (word anchors): ‘not at all bothersome’ (0mm) and ‘intolerably bothersome’ (100mm).
At Visit 2, a Global Rating Scale (GRS) of seven points was completed to evaluate any change in health status (Wyrwich KW, Wolinsky FD. Identifying meaningful intra-individual change standards for health-related quality of life measures. J Eval Clin Pract 2000;6(1):39e49). It is a seven-point Likert-type scale scored by the patients from “large deterioration” (−3) to “large improvement” (+3), in which “0” means “no change”.
The psychometric properties of the Spanish version of RAPP were tested by means of the following assessments:
- 1)
Internal consistency of RAPP items using Cronbach’s alpha coefficient. Values >0.70 are generally considered acceptable,23 whereas higher scores are recommended for use in an individual patient.24
- 2)
Scale reliability by mean of interclass coefficient (ICC) and Lin’s concordance correlation coefficient (CCC),27 assessed in a subsample of patients with a stable health status (GRS=0). Coefficients of 0.70 for group comparisons, and of 0.90 for comparisons within individuals are recommended.25
- 3)
Scale dimensionality was determined using explorative and confirmative factor analysis.26,27 The root-mean-square error of approximation (RMSEA), the comparative fit index [CFI], and the standardized root mean squared residual (SRMR) were used to assess the confirmative model fit.
- 4)
Convergent validity was evaluated using Pearson’s correlation between the RAPP and SF-12 scores. Correlations ranging from 0.4 to 0.8 were considered as an indication of convergent validity.
- 5)
Discriminant validity was assessed by mean of ANOVA (Fischer’s test) comparing groups of patients derived from the ACT, GINA and ARIA classification of severity.
- 6)
Responsiveness was evaluated by analyzing the correlation between changes in RAPP scores and changes in GRS, VAS and ACT by means of Pearson correlation coefficients.
- 7)
The receiver operating characteristics (ROC) curve method was applied to determine the minimal important difference (MID). The entire cohort for one dichotomization point (i.e., ‘no change’ vs. ‘any improvement or deterioration’) was adopted.28
The possible effect of age was explored by means of correlation analysis, while the education and smoking habits on patients’ answers were controlled using ANOVA (Fisher’s Test).
The frequency distribution of RAPP scores was calculated to check whether they covered the entire scale, i.e. if patients had heterogeneous quality-of-life levels.
IBM SPSS Statistics (version 24, Armonk, NY) and Mplus 7.0 (Muthén & Muthén, Los Angeles, CA, USA) were used to perform the statistical analysis.
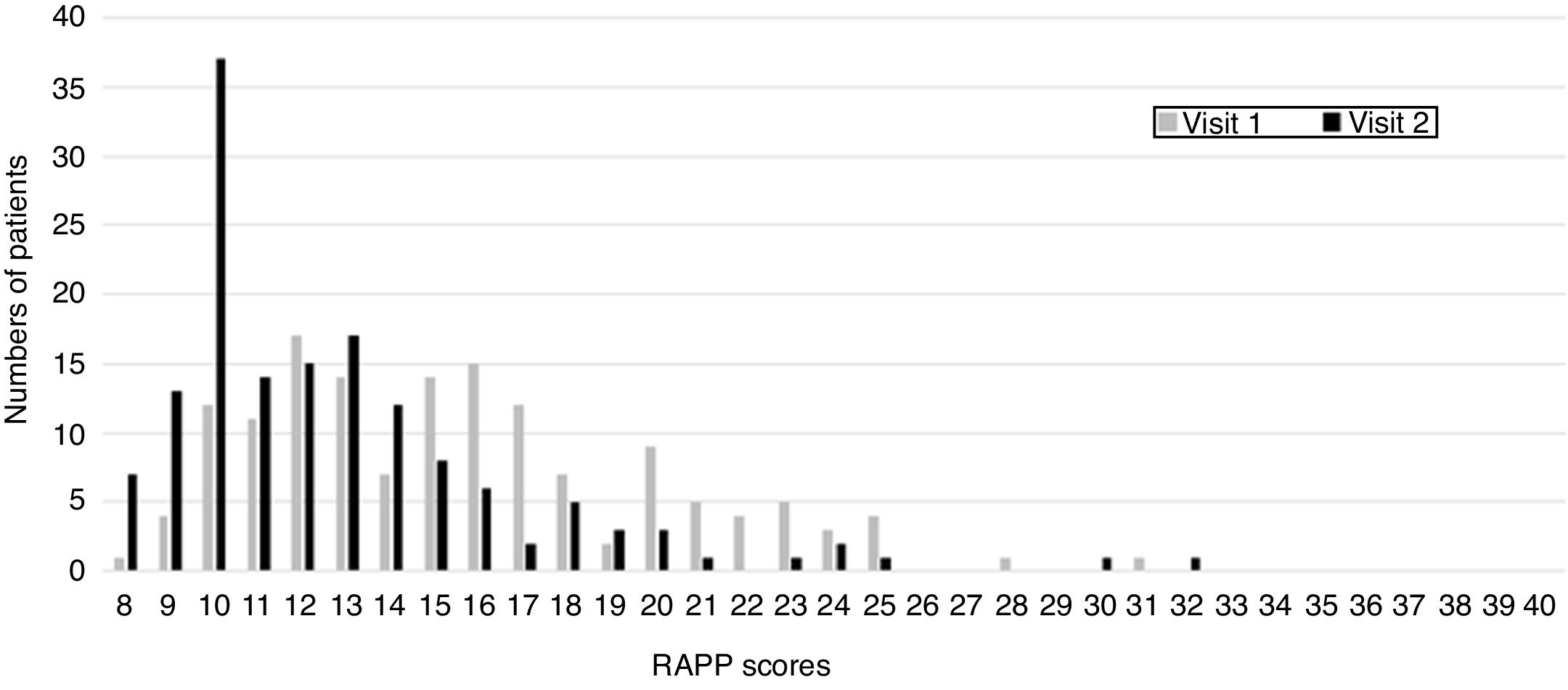
Results149 patients were included in this study; the mean age was 37.7 years (SD 10.3, range 18–76), and the majority were females (62.8%). The most common level of education attained was Academic degree (73.6%), followed by high school (15.5%), secondary school (9.5%) and primary school (1.4%) degrees. 12.3% of patients were students, 79.5% workers, 8.2% unemployed and none were retired. Intermittent asthma was prevalent in the sample (57.9%). At the first visit, ACT scores indicated that 61.7% were totally controlled, 22.1% were well controlled, and the remaining 16.1% were uncontrolled. The mean value of AR and asthma quality of life was 15.6+4.5 at visit 1 and 12.8+4.6 at visit 2. The distribution of answers for the first and second visits are presented in Fig. 1.
Cronbach’s alpha values of 0.73 were obtained at the first visit and 0.83 at the second, both indicating a satisfactory internal consistency. Reliability analysis, assessed in a subsample of 40 patients reporting stable health status (GRS=0), provided an almost acceptable ICC of 0.69, while the CCC=0.74 resulted quite low, indicating that comparisons between patients should be performed with caution. Exploratory factor analysis revealed a unidimensional structure that absorbed 33.3% of the total variance and only five residuals greater than |0.10| at the first visit and 41.1% of the total variance and only three residuals greater than |0.10| at the second visit. The fit indexes of the confirmatory factor analysis were all satisfactory, both at the first (RMSEA 0.06, SRMR 0.06, CFI 0.93) and second visits (RMSEA 0.07, SRMR 0.06, CFI 0.91) supporting the unidimensional structure of RAPP. Pearson correlations between RAPP scores and the Physical Component Scores of SF-12 were significant both at first (r=−0.39, p=0.000) and second visit (r=−0.62, p=0.000), denoting convergent validity. Significant correlations were also found between RAPP and the Mental Component Score of SF-12 both at Visit 1 (r=−0.28, p<0.001) and at Visit 2 (r=−0.38, p=0.000).
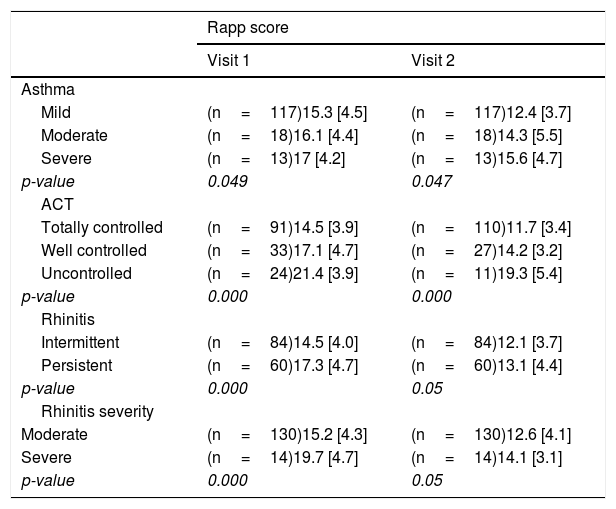
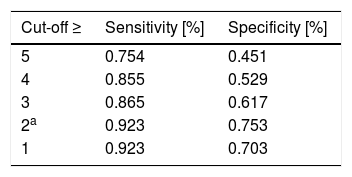
The analysis of Discriminant validity, presented in Table 1, showed that RAPP is able to discriminate between patients on the basis of asthma control level and rhinitis severity (p<0.05 for all the analyses). Responsiveness: the assessment of a subsample of 109 patients reporting an improvement or deterioration in health status (GRS ≠ 0) demonstrate that changes in RAPP were significantly associated with changes in VAS (r=0.28, p<0.003) and ACT (r =−0.35, p=0.000), and not significantly associated with changes in GRS (r=0.11, p=0.218). The MID resulted equal to a 2-point difference or change in RAPP score, and was sufficient to maximize sensitivity, specificity, and the number of individuals correctly classified (Table 2).
RAPP discriminant validity (means and standard deviation).
| Rapp score | ||
|---|---|---|
| Visit 1 | Visit 2 | |
| Asthma | ||
| Mild | (n=117)15.3 [4.5] | (n=117)12.4 [3.7] |
| Moderate | (n=18)16.1 [4.4] | (n=18)14.3 [5.5] |
| Severe | (n=13)17 [4.2] | (n=13)15.6 [4.7] |
| p-value | 0.049 | 0.047 |
| ACT | ||
| Totally controlled | (n=91)14.5 [3.9] | (n=110)11.7 [3.4] |
| Well controlled | (n=33)17.1 [4.7] | (n=27)14.2 [3.2] |
| Uncontrolled | (n=24)21.4 [3.9] | (n=11)19.3 [5.4] |
| p-value | 0.000 | 0.000 |
| Rhinitis | ||
| Intermittent | (n=84)14.5 [4.0] | (n=84)12.1 [3.7] |
| Persistent | (n=60)17.3 [4.7] | (n=60)13.1 [4.4] |
| p-value | 0.000 | 0.05 |
| Rhinitis severity | ||
| Moderate | (n=130)15.2 [4.3] | (n=130)12.6 [4.1] |
| Severe | (n=14)19.7 [4.7] | (n=14)14.1 [3.1] |
| p-value | 0.000 | 0.05 |
The minimal important difference (MID) of RAPP obtained with the ROC analysis with different cut-offs.
| Cut-off ≥ | Sensitivity [%] | Specificity [%] |
|---|---|---|
| 5 | 0.754 | 0.451 |
| 4 | 0.855 | 0.529 |
| 3 | 0.865 | 0.617 |
| 2a | 0.923 | 0.753 |
| 1 | 0.923 | 0.703 |
RAPP scores showed no significant differences between smokers, former smokers, and non-smokers (Fisher’s test, Visit 1: p=0.07; Visit 2: p=0.39), neither as regards age (Pearson correlation, Visit 1: r=0.018; Visit 2, r=−0.06) nor in terms of level of education (Fisher’s test, Visit 1: p=0.21; Visit 2: p=0.27).
DiscussionThere is growing interest in integrating HRQoL in the clinical setting29 to provide clinicians with standardized tools to capture the patient’s viewpoint of their disease and treatment.30,31 The only available tool for the routine assessment of HRQoL in patients with respiratory allergy is the RAPP,6 which was originally developed and validated in Italian.32
In this study, we report the results of the cross-cultural adaptation and validation of the RAPP into the Spanish language.
The process of translation and adaptation followed the recommended procedure.12,13 No critical aspects or content-related problems were observed in this process. The Spanish version was free of cross-cultural inconsistences.
Using the same approach as for the original RAPP, the results confirmed the unidimensional structure and the validity of the Spanish version. The high Cronbach’s alpha coefficient [0.73 at visit 1 and 0.83 at visit 2] indicates a satisfactory level of internal consistency. As expected, a moderate, significant correlation was obtained between RAPP and SF-12 scores. In fact, the SF-12 provides an assessment of the physical and mental components of health status, which are two domains of HRQoL.31 Discriminant validity was demonstrated through the tool’s capacity to discriminate between groups defined according to the level of control and disease severity.
The Spanish RAPP has been shown to be responsive and the change significantly correlates with variation in measures of asthma control and AR severity.
The threshold of 2 points of MID found for the Italian6 and the Portuguese7 RAPP was also confirmed for the Spanish version.
There were some limitations to this study. First of all, due to the observational nature of the study, responsiveness was not evaluated by means of a before-and-after interventional design. Second, no objective measures of disease control and severity, besides the patient’s reported outcomes, were used to determine the sensitivity to change.
ConclusionsThe Spanish version of RAPP has been shown to be a valid tool with satisfactory measurement properties and can therefore be used for the assessment of HRQoL with the individual patient.
Our results represent the first step to perform replication studies to confirm the validity of the Spanish RAPP to other Spanish-speaking populations.11,12
FundingThis work was supported by Menarini International Operations Luxemburg S.A. [M.I.O.L.]
Conflict of interestThe authors have no conflict of interest to declare.
The authors thank ARMIA [Associazione Ricerca Malattie Immunologiche e Allergiche] for scientific support. This work was supported by Menarini International Operations Luxemburg S.A. (M.I.O.L.). Contract ARMIA M.I.O.L.19/09/2016.