INTRODUCTION
Corticosteroids (CS) regulate a number of physiologic processes, including development, stress responses, and homeostasis, and also have a significant interaction with the immune system1. These activities have important therapeutic consequences, and today CS are indispensable for the treatment of a wide variety of inflammatory diseases. A range of adverse effects limits their systemic use; however, inhaled (ICS) and intranasal (INS) corticosteroids play a pivotal role in the treatment of asthma and allergic rhinitis.
The chronic inflammatory processes associated with increased expression of multiple inflammatory genes, are regulated by pro-inflammatory transcription factors such as nuclear factor-kappa beta (NF-κβ) and protein activator-1 (AP-1). These transcription factors bind and activate coactivator molecules (CBP, SRC-1, TIF-2, p300/CBP) that acetylate histones (protein components of chromatin), inducing gene transcription of inflammatory cytokines2.
In spite of the ability of CS to induce gene transcription, the major anti-inflammatory effects of CS are through repression of inflammatory and immune genes.
MOLECULAR MECHANISMS OF CORTICOSTEROIDS ACTION
The anti-inflammatory action of CS is measured by their binding affinity to glucocorticoid receptors (GR) in the cytoplasm. CS has ability to diffuse across cell membranes into cytosol and bind to the receptor site. Cytoplasmic GRs generally bind to carrier proteins such as heat shock 90-kDa proteins (hsp90) and FK-binding protein that protects the receptor and prevents it from being confined in the nucleus3.
Once bound to the GR, CS undergoes structural changes that lead to dissociation of carrier proteins, exposing nuclear localization signals to GR. This results in quick transport of CS/GR complex into the nucleus, where the complex binds to specific DNA sequences in the gene promoter region (GRE). After binding to receptors in DNA, CS can promote or inhibit gene expression through processes called transactivation and transrepression, respectively. For example, CS transactivate the beta-2 adrenergic receptor gene, the lipocortin-1 gene, the interleukin (IL)-10 gene, and the NF-κβ(Iκβ -α) inhibitor gene with anti-inflammatory actions. CS also promotes the synthesis of two proteins that affect the inflammatory signal transduction pathway: glucocorticoid-induced leucine-zipper protein (GILZ), which inhibits NF-κβ and AP-14, and MAP kinase phosphatase-1 (MKP-1), which inhibits p38 MAP kinase.
Meanwhile, most of the genes that are transactivated by CS are likely to be involved in side effects, including hypertension, edema, hypocalemia, glaucoma, and diabetes5. Through mechanism of transrepression, CS "inhibits" the action of transcription factors AP-1 and NF-κβ decreasing the production of inflammatory mediators, possibly by inhibiting histone acetylation (HAT)2.
In inflammatory diseases there is an increase in HAT activity and some decrease in histone deacetylation (HDAC) activity, which is restored by the treatment with CS. CS inhibit the transcription of various cytokines and chemokines that are relevant to inflammatory lung diseases, including IL-1β, TNF-α, GM-CSF, IL-4, IL-5, IL-8, and eotaxine1. It is accepted that this is the most important mechanism of action of CS on inflammatory diseases. Not only does CS block the synthesis of cytokines, they also block cytokine effect by inhibiting the synthesis of cytokine receptors such as the IL-2 receptor.
As the cell genome is involved in this mechanism, this anti-inflammatory effect is alternatively referred to as a genomic effect. In terms of response, after CS molecule enters the cell, hours or even days may elapse before significant quantities of new proteins are produced. This explains the 6 to 12 hours' delay (demonstrated by clinical trials6) in detecting the beneficial action of systemic CS.
More recently, however, it has been demonstrated that CS have biological effects that are independent of the gene transcription process7. A recent research has centered on the nongenomic effects of inhaled CS on the airways, most particularly on mucosal blood flow in both asthmatic and healthy subjects8. CS has also been shown to acutely decrease nasal itching in allergic rhinitis patients9. These rapid effects are initiated by specific interactions with membrane-bound or cytoplasmic GRs, or nonspecific interactions with the cell membrane10, and the responses are faster (seconds or minutes).These studies show that there is a significant increase in mucosal blood flow in asthmatic patients compared to healthy subjects, and that ICS has the effect of reducing flow by causing vasoconstriction11 enhancing norepinefrine action during synapsis between sympathetic endings and smooth muscle cells in the mucosal vasculature12.
To sum up, CS have a dual effect on asthmatic patients13. In particular, the nongenomic effect occurs within minutes, is transient, depends on the dose administered, and is proportional to the initial hyperperfusion level. These fundamental features of CS use should be taken into account when administering ICS to patients with severe asthma.
Beyond immunosuppressant and anti-inflammatory properties, CS promotes the differentiation of regulatory T cells (CD25+ CD4+) through a FOXP3-dependent mechanism. The regulatory CD25+ CD4+ T cells represent a population of lymphocytes capable of suppressing the immunological response. FOXP3 marker is correlated with the expression of the anti-inflammatory cytokine IL-10, and is a marker of the activation of regulatory T cells14.
CLINICAL EFFICACY OF INTRANASAL OR INHALED CORTICOSTEROIDS
INS represents the single most effective class of medicines for allergic rhinitis and improves all nasal symptoms, including nasal congestion, rhinorrhea, itching, and sneezing. Most studies15,16 but not all17 have shown that treatment of rhinitis with INS also leads to decreased methacholine sensitivity of the lower airways, better asthma control18, and fewer asthma-related emergency room visits19. Currently there are available for rhinitis treatment: beclomethasone dipropionate (BDP), budesonide (BUD), fluticasone propionate (FP), mometasone furoate (MF), and triamcinolone acetonide (TAA). The purpose of development research is to discover new products with enhanced benefit-to-risk profiles. Although INS may vary in their sensory attributes (eg, taste or smell) and thus in degree of patient acceptance and adherence, there do not appear to be any clear, clinically relevant differences in efficacy among them20.
Ciclesonide (CIC) is an investigational CS under development for treatment of allergic rhinitis. Intranasal CIC treatment has been associated with significant reductions in nasal symptoms and appreciable improvements in health-related quality of life in adult and adolescent patients with persistent allergic rhinitis21. The fluticasone furoate (FF) is the last generation of INS that it will be placed soon in the international market.
ICS play a pivotal role in the treatment of asthma because they exert a local effect at the site of action and thus decrease the risk for adverse reactions. There are available currently for asthma treatment: BDP, BUD, FP, MF, and CIC.
The clinical efficacy of ICS is dependent on asthma severity and duration, treatment regimens (duration, dose, drug etc..), and on exposure to allergens and infectious agents during the study. Significant improvement in lung function (forced expiratory volume in the first second [FEV1] and peak expiratory flow [PEF]), reduction in number of asthma exacerbations, decrease in asthma symptom score, and reduction of rescue inhaled short-acting beta-2 agonists in comparison to placebo were recently associated to inhaled BDP, BUD, and FP regimens in three systematic literature reviews22-24.
Usually, symptoms of asthma show a clear improvement after a few days, whereas maximum improvement of lung function may require weeks, maybe months. Furthermore, maximum improvement of bronchial hyper-responsiveness (BHR) may take months after the treatment with CS is begun, and this benefit goes gradually reducing with the withdrawal of the drug, especially in moderate and severe asthma. This suggests that topic CS are unable in changing the natural history of allergic diseases and that treatment should be adjusted to the minimum dose capable of promoting clinical stability.
The efficacy of ICS or INS depends on the topical activity of the drug that reaches the lungs or nasal mucosa respectively, while the adverse effects depend on oral deposition and on systemic activity. The drug's systemic activity depends on the amount absorbed by both the gastrointestinal tract and the lungs.
The amount of ICS delivered to the lungs depends on inhalation technique, the type of inhaler used, the solvent, the propellant, the size of delivered particle, and on whether or not spacers are used25. The fine particle dose of the drug is defined as the fraction of particles with a diameter between 1 and 4 mm26. These small particles penetrate more deeply into the lung and thereby, more effectively dilate the small airways than larger particles, which are filtered out in the upper airways. Any one drug may have a number of different formulations and be packaged with various delivery devices.
Each inhalation device, whether a nebulizer, a dry powder inhaler (DPI) or a metered-dose inhaler (MDI) generates its drug aerosol differently and thus, the particle size, respirable dose, lung deposition and distribution will also differ. Hydrofluoroalkane-134a (HFA) has been shown to be a safe replacement for chlorofluorocarbons (CFCs) as a pharmaceutical propellant, with the advantage that it has no ozone-depleting potential and a superior lung deposition, reaching particularly the small airways27. The use of spacer devices can alter the amount of drug in the respirable range and decrease the amount of drug deposited in oropharynx and swallowed, thus altering both therapeutic efficacy and potential for systemic effects28. Consequently, the same drug at the same nominal dose delivered from different devices or in different formulations may not be bioequivalent29.
A meta-analysis of 14 comparative clinical trials demonstrated that half dose of FP (as compared to BUD and BDP) was numerically superior in all trials and statistically superior in four of them when compared with BUD and BDP. Therefore, despite the difficulties with standardization, the trials suggest that when using pMDI, FP is more effective than BDP, TAA, and BUD; however, the efficacy of BUD in turbuhaler device is similar to that of FP delivered by pMDI or by diskhaler, and better than that of BDP30.
We should all do our best to spend a few extra minutes with our young patients and their parents to ensure that the drugs we prescribe are delivered in the best possible manner. This means improving asthma control, reducing side effects and offering a more cost-effective therapy. Advice on the appropriate use of spacers should include proper agitation, correct timing of the actuation of the pMDI, single actuations and not multiple pMDI actuations and correct spacer care31,32. That is important to have a proper fit of patient and device to obtain optimal benefit compared with risk of adverse effects for the individual patient.
Inhaled MF delivered by DPI is effective in treating patients with persistent asthma. It improves pulmonary function and health-related quality of life, reduces symptoms and decreases oral CS requirements in severe disease. It is a potent anti-inflammatory agent and is at least as clinically effective as other ICS. Inhaled MF is equally effective in controlling asthma when administered in two divided doses or as a single daily dose33.
CIC has potent inhibitory effects on features of chronic allergic pulmonary inflammation, airway remodeling, and in bronchial hyper reactivity at doses that did not change body weight and hypothalamic-pituitary-adrenal axis34. CIC is formulated as a solution for delivery via HFA-MDI, which results in high lung deposition. Recent studies demonstrate comparable efficacy with other ICS in patients with persistent asthma35,36 and, in addition, CIC is associated with minimal local or systemic adverse effects37,38.
All commercially available ICS or INS have potentially similar efficacy (ie, all agents can achieve the maximum response on a dose-response curve). However, because of significant differences in pharmacokinetic and pharmacodynamic properties, their potencies (the amount of CS needed to achieve maximum response) differ greatly39. Although these differences may not affect efficacy, they may affect safety (therapeutic ratio) and convenience and are a crucial consideration when comparing different ICS or INS preparations40.
POTENCY OF THE INHALED OR INTRANASAL CORTICOSTEROIDS
It is difficult to compare the absolute potency levels of the various ICS considering that the available have not been compared in a single study. The potency of a CS or its capacity to produce a pharmacologic response is based on its relative potency determined by various measures such as cutaneous vasoconstriction assays (human skin blanching), receptor binding affinity, lipophilicity, and inhibition of inflammatory cells, mediators, and cytokines. Available in vivo and in vitro measurements of CS functional activity suggest the following relative potencies: MF and FP > BUD = BDP > TAA = flunisolide (FLU)41. From a pharmacological point of view, the differences in potency are relatively insignificant unless they translate into clinical efficacy.
The activity of a drug depends on its pharmacokinetic and pharmacodynamic properties, and the characteristics of each inhalation device used (e.g. distribution of particle size, efficacy of pulmonary delivery, and convenience for use). However, the therapeutic index, or clinical efficacy, is the only measurable parameter for comparing new ICS or INS with the previous ones.
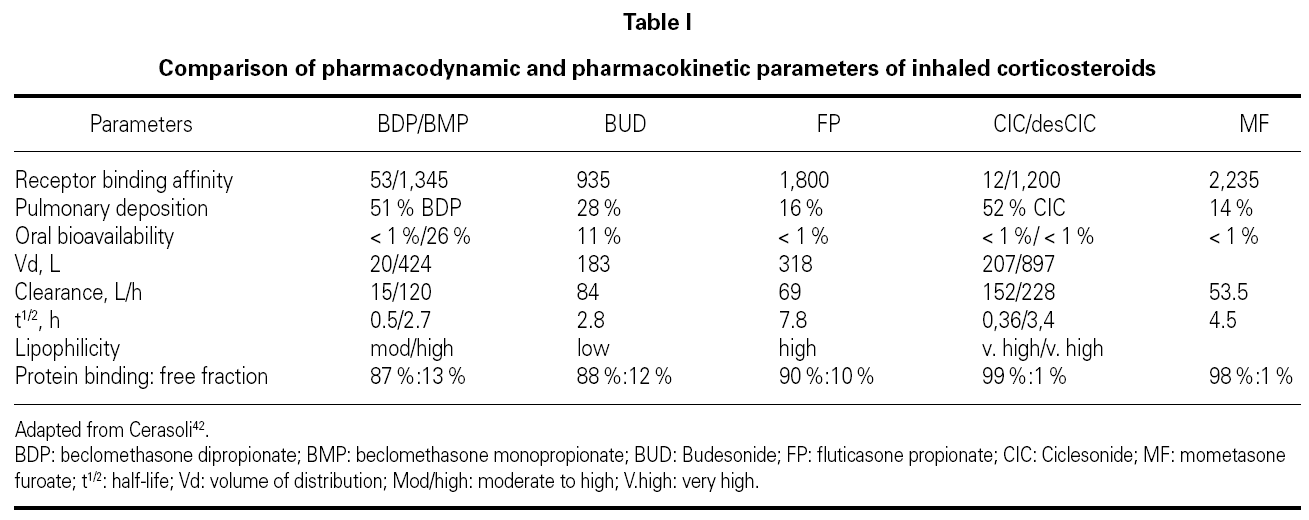
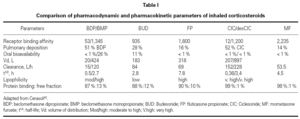
Since same receptor mediates the effects of all ICS, the qualitative response resulting from the binding to GR is similar for all. Therefore, the pharmacodynamics of ICS and INS depends exclusively on receptor affinity. The binding ability of inhaled glucocorticoids is expressed by the receptor affinity compared with dexamethasone. Dexamethasone has a binding affinity of 100. The higher the binding affinity, the lower the concentrations that induce an effect. In order to ensure equivalent effects, the differences in affinity can be compensated by controlling the dose, that is, the concentration of the drug at the GR binding site. Since the pharmacodynamics of each ICS and INS depends only on the drug's relative GR binding affinity, and because this difference in affinity can be controlled by dose adjustments, the greatest difference between these different CS should be due to their pharmacokinetic properties (table I)42.
The following aspects related to the pharmacokinetics of ICS and INS are considered to be important: bioavailability, volume of distribution, clearance, half-life, lipophilicity, protein binding, and nature of the CS under consideration (biologically active drug or pro-drug).
BDP and CIC are the two agents that can be distinguished from the other topic steroids because they are prodrugs. These drugs are not active in their native form; they need to be activated by metabolic reaction. CIC, considered a soft steroid, is activated after being cleaved by specific mucosal esterase present in the lung and nasal mucosa, which ensures fewer adverse effects43. It is a pro-drug without direct activity and low affinity for GRs. Activated CIC is quickly metabolized and transformed in inactive products42. BDP is metabolized in the lung to 17- BMP, 21-BMP and beclomethasone. 17-BMP has the highest affinity for glucocorticoid receptors and it is known to circulate at greater concentrations in the serum compared with other metabolic breakdown products44.
Although ICS and INS are applied topically, a significant portion can be absorbed systemically. Bioavailability is the amount of drug that reaches the systemic circulation. Systemic bioavailability is the sum of two components, including the portion of the drug that is swallowed plus the portion of the drug that is absorbed via the pulmonary or nasal mucosa The goal of topic steroids design is to achieve a high ratio of topical to systemic activity39.
In order to reduce systemic adverse events of these drugs, they should be eliminated from the systemic circulation as quickly as possible. All ICS and INS are quickly metabolized by the liver (~90 L/h). Volume of distribution (Vd) is the fluid volume required to contain the entire drug at the same concentration existing in the blood and is a measure of relative tissue uptake. Drugs that are primarily present in tissues have low serum concentrations and therefore large Vd, while the drugs that are primarily present in the blood present low Vd. FP and the two active pro-drug metabolites present large Vd, which means good tissue penetration, in this case into lung tissue (table I)45.
Clearance is the rate of elimination by all routes relative to the concentration of drug in the blood and is a measure of the elimination capacity.
Half-life is the time required for the drug concentration to drop by 50 %. Drugs with high clearance have short half-lives, and drugs with large Vd have longer half-lives45. Another way of measuring how long the CS stays in the lung (pulmonary residence time) is by calculating the percentage of the drug absorbed over time. Consistent with its long half-life, FP is absorbed slowly, with a significant amount remaining in the lungs 4 to 8 hours after inhalation. In contrast, BUD quickly disappears in the lungs (table I).
Lipid conjugation is another important parameter to evaluate ICS' pharmacokinetics. Lipid-conjugated ICS is retained in the lungs and is not absorbed by systemic circulation. The distinction between lipid conjugation and lipophilicity is important. Drugs with high lipophilicity frequently present a high degree of unspecific binding to lipids and proteins, which results in their widespread distribution in tissues. As a result of the large Vd, drugs such as FP, which have high lipophilicity, also have a long half-life (table I).
Protein binding is important because only CS-free molecules can interact with GR; protein-bound molecules are inactive. BDP, BUD, and FP have similar percentages of free drug (~10 %). The active product of CIC (des-CIC) has a protein-binding level greater than 99 %, which results in a very low proportion of free drug in circulation in comparison to other ICS. As a result of this high protein binding, less than 1 % of des-CIC entering the systemic circulation is available for potential adverse systemic effects, in comparison to 10 % or more for other inhaled CS. Therefore, CIC produces significantly less suppression than other ICS46 (table I).
DOSE VS. SAFETY
The ideal CS should have potent topical activity with minimal adverse effects and no systemic adverse effects.
All topic CS, after be delivered, are absorbed systemically and have dose-related adverse systemic effects. Systemic absorption can occur directly through the lung surface (ICS) or nasal mucosa (INS) and by swallowing the drug.
In asthma, ICS delivered dose that reaches the lungs, after the pMDI activation, is approximately 10 to 20 % of the nominal dose. Remain amount deposited on the oropharynx will be swallowed and subsequently absorbed through the gastrointestinal tract. The dose of ICS delivered to the lungs will also be transferred to the systemic circulation. Absorption through the lung surface is quick, and if the drug is not locally metabolized there could be extra-pulmonary effects, especially with very high doses.
Concerning to INS, more than 50 % of the nominal dose delivered through the nasal pump spray will be deposited on mouth, swallowed and posteriorly absorbed through the gastrointestinal tract. Immediately after its absorption, these drugs will be inactivated during its first-pass through the liver before entering the systemic circulation. Some of these INS, especially MF and FP are extensively metabolized during their first passage through the liver. Therefore, after oral absorption, they enter the systemic circulation as inactive metabolites39. Those INS that are not efficiently inactivated during first-pass metabolism, will gain the systemic circulation without modifications, resulting in extra-pulmonary side effects.
Regarding nasal absorption, it is reasonable to expect that a high dose of these drugs would reach the systemic circulation due to its high level of absorption through the abundant vascularity of the nasal mucosa47.
ADVERSE EFFECTS
The topical route of administration improves targeting of CS to the upper and lower airways so that high local concentrations of drug are achieved with less systemic exposure and less adverse systemic effects. Topical administration may, however, lead to local adverse effects, including oral candidiasis, hoarseness and dysphonia following oral inhalation, and dryness, crusting, and bleeding with intranasal use48. CIC offers a significantly lower chance of local side effects since it is not activated on the oral mucosa.
The use of devices (spacers) can also promote less oropharyngeal deposition48.
For topical administration to be effective, airways proximal to the inflammation need to be patent. An INS is unlikely to be beneficial when the nasal passages are blocked, and the same may be true for INS when there is marked airflow obstruction.
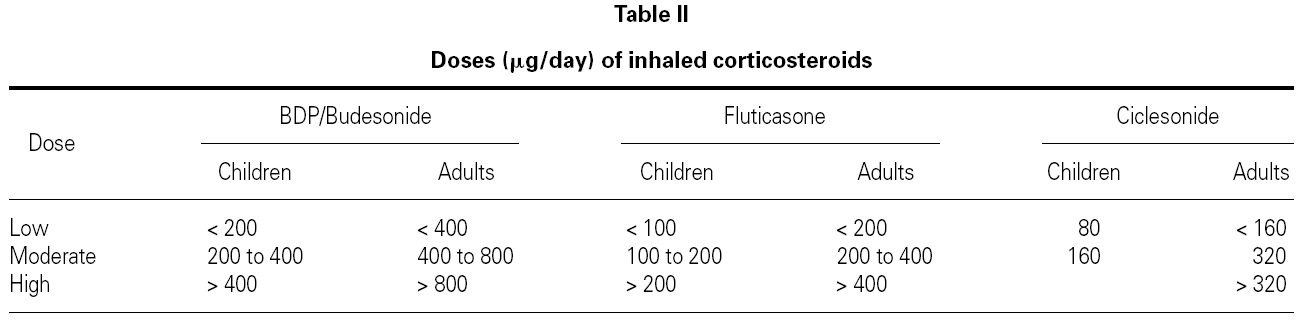
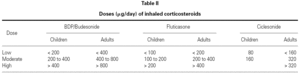
Dose, duration and dosing schedule of CS treatment are clearly important determinants of the benefit/risk ratio. Evidence shows that for most patients who have asthma, much of the benefit of ICS is obtained with fairly low doses49. Meta-analyses of placebo-controlled published studies have suggested that most of the therapeutic benefit in asthma is achieved with doses of around 400 mg/d for BUD and 200 mg/d for FP, at least for change in lung function50,51.
The standard doses of ICS for adults and children are listed in table II.
Besides the advantage of topical applications in the lower occurrence of adverse systemic effects, all topical CS are systemically absorbed and have a class effect of dose-dependent adverse effects.
The main adverse systemic effects of the topical CS are as follows: hypothalamic-pituitary-adrenal axis suppression, bone mineral density, growth, and ocular toxicity (including subcapsular cataract and glaucoma)42,52.
HYPOTHALAMIC-PITUITARY-ADRENAL AXIS SUPPRESSION
The frequency of secondary adrenal insufficiency due to suppression of hypothalamic-pituitary-adrenal (HPA) axis resulting from ICS treatment is very low. Few cases in children have been associated with long-term treatment with FP53. There is no consensus regarding the suppressive action of ICS on the HPA axis, and the method used to evaluate this suppression is one of the factors that can affect the interpretation of results. Suppression can be evaluated by 24-hour serial monitoring of serum cortisol levels, by determination of nocturnal or 24-hour urine cortisol, and by adrenocorticotropic hormone (ACTH) stimulation test. Further confounding factors are the equivalence of the ICS doses used and the devices used.
A meta-analysis study carried out with adults and children concluded that inhaled FP has significantly greater adrenal suppressing potential when compared to inhaled BDP, BUD or TAA54.
Patients in treatment with a low to moderate dose of ICS (< 400 μg/day of BDP, BUD or TAA, or < 200 μg PF, or 160 μg of CIC) usually do not present significant changes in 24-hour plasma cortisol levels55, in urinary cortisol, and in the response to ACTH stimulation test56. However, suppression of the HPA axis has been detected (without any clinical expression) when using powder inhalation devices, which increase the amount of drug that reaches the lung even in these lower doses57.
CIC, the most recent ICS available for clinical use in children, has demonstrated efficacy in asthma treatment and a better profile of side effects when compared to other CS58. Because of its high sensitivity to hepatic oxidases, CIC has a very short plasma half-life, which reduces systemic exposure to the active drug to a minimum42. This low systemic exposure has been shown in recent studies demonstrating the absence of a relevant clinical effect on the HPA axis even with high doses, such as 320 to 1,280 μg59.
In conclusion, treatment with low or moderate doses (< 400 μg/d) of ICS is usually not associated with suppression of the HPA axis in children. Because of this, the routine monitoring of the HPA axis is not necessary, unless there is evidence of growth suppression. On the other hand, children with chronic asthma who receive high doses of ICS or who have been receiving CS through other routes (topical, intranasal) should have their morning levels of plasma cortisol monitored periodically, even in the absence of increased risk of HPA axis suppression. In the presence of low levels, they should be submitted to the ACTH stimulation test45.
Regarding to INS, FP (220 μg/day) has been reported to suppress HPA axis activity by reducing the overnight urinary cortisol levels in comparison to TAA at same dosage60,61. According to the authors it would be due to the enhanced FP lipophilicity. On the other hand, BUD aqueous nasal spray for 6 weeks, and FP aqueous nasal spray did not show any interference on HPA axis in children from 2 to 5 years old62,63.
BONE METABOLISM
Because CS increase reabsorption and decrease bone formation, they can cause dose- and age-dependent osteoporosis. Bone turnover is greater in children than in adults. Bone mass/density acquisition begins in childhood and peaks in young adults. Many factors are identified as capable of interfering with the content of bone mass: sex, nutrition, hereditariness, endocrine factors, and physical activity.
The effects of exogenous CS on bone can be evaluated by biochemical markers of bone metabolism, bone mineral density (BMD), or frequency of fractures. A recent review of ICS effects on bone showed no evidence of changes in bone markers or degradation in children treated with ICS in standard doses64. Moreover, elevated doses may cause significant changes in the bone turnover rate, but the occurrence of these changes during the treatment, which is usually short-term, deserves further studies52.
Asthmatic children treated with BUD (> 800 μg/ day) for longer than 18 months, or BUD (500 μg/day) for 4.5 years, or BDP (300-800 μg/day) for 2 years do not present reduction of BMD when compared to those treated with placebo or smaller doses of the respective ICS65-67. In wheezing infants, the use of an intermittent treatment model with inhaled BUD (400 mg/day) did not determine significant changes in BMD68. In a recent review of the use of ICS in children with asthma, none of the four trials evaluating BMD, presented a significant alteration69.
In light of the current studies, there is no evidence that the long-term treatment of children with ICS in low doses is associated with the reduction of BMD or with increased risk of osteoporosis or fracture70. However, changes in the total bone mineral content in children treated with high doses of BDP or BUD or FP have been recently documented during 12 months of treatment71. An experimental assay has documented the absence of effect on bone metabolism with CIC, even in elevated doses46. There are not enough data available for INS administration and its effects on bone metabolism72.
LINEAR GROWTH
Growth is a complex, non-homogenous physiological phenomenon that is influenced by many factors: genetics, nutrition, hormones, and others. CS interferes with collagen turnover and with somatomedin levels, the final growth promoter, produced by human growth hormone; therefore, these drugs may be associated with growth deficit in children with asthma and long-term treatment with ICS, especially in high doses45. This interference is more evident during fast growth phases (spurts) in preschool years and puberty. Asthma, however, in and of itself, can interfere with the growth rate.
To monitor growth rate, knemometry (measurement of lower leg length) is useful to detect changes occurring over a short period of time and stadiometry is useful to detect changes over medium or long-term periods. However, adult stature is the most adequate parameter73. Current evidence shows that treatment with ICS (medium/high doses) can induce delay in the growth rate at the start of treatment with BDP or BUD22,23,45,74. However, this interference is transitory, since there are no reports of an influence on the adult stature of these patients45. A few patients receiving higher doses of BDP or BUD (> 750 μg/day) during 14 weeks presented growth retardation. According to the United States National Asthma Education and Prevention Program, low or medium doses of ICS have the potential to impact growth rate, but the effects are small, non-progressive, and possibly reversible. Furthermore, the adult height reached by asthmatic children with ICS treatment is not different than that reached by non-asthmatic children75.
A meta-analysis of 21 trials including 810 patients has compared the stature reached in relation to the treatment with ICS or oral CS. There was slight growth impairment in those treated with oral CS76. The Childhood Asthma Management Program (CAMP) compared the efficacy and safety of long-term treatment (4 to 6 years) of BUD and nedocromil sodium in children with mild to moderate asthma. Treatment with BUD resulted in improved airway reactivity, better control of asthma, and transitory reduction in growth rate. A similar finding was reported by other investigators77,78.
Treatment with FP was evaluated in children with mild asthma, and no interference was observed. On the other hand, Guilbert et al. evaluated 2 years of treatment with FP (176 μg/day) in children aged 2 to 3 years old. In addition to clinical control during the active treatment period, a reduction in growth rate, with partial recovery during the follow-up period, was also recorded79. A recent double-blind, placebo-controlled study with children treated with different doses of inhaled ciclesonide did not document changes in either lower leg growth rate or effects on the HAP axis80,81.
In patients with allergic rhinitis a long-term study with BDP (low dose) was associated to growth retardation. It was not associated with MF82 and FP61 long-term treatment.
OCULAR TOXICITY
The risk of subcapsular and nuclear cataract associated with the use of ICS is not significant in pediatric patients with asthma and/or allergic rhinitis; however, it may be greater in the elderly. Sufficient information concerning the differences in the risk of cataract associated with the different ICS formulations is not available45,64.
QUALITY OF LIFE
Although topical CS does not modify natural evolution of allergic diseases, there are great advantages in their use, improving patient's quality of life. Sleep quality can be significantly impacted by nasal congestion, a common symptom related to allergic rhinitis. This may lead to decreased learning ability, productivity at work or school, and a reduced quality of life.
ICS and INS improve performance at school and at work, and reduce sleep disturbances associated with breathing symptoms83-85.
They are more effective when begun days before the exposure to allergens or irritants and should be used regularly, for periods of time and in enough doses to keep the patient clinically stable.
CS RESISTANCE IN ASTHMA
Although CS are highly effective in the control of asthma and other chronic inflammatory or immune diseases, a small proportion of patients with asthma fail to respond even to high doses of oral glucocorticoids86. CS resistance in asthma is not absolute, and patients often respond to very high doses of inhaled and/or oral CS. The reduction in CS responsiveness observed in some individuals has been ascribed to a reduced number of GRs, altered affinity of the ligand for GRs, reduced ability of the GRs to bind to DNA, or increased expression of inflammatory transcription factors, such as AP-1, that complete for DNA binding87,88.
Defects in ligand binding
Certain cytokines might induce a reduction in the affinity of GRs in inflammatory cells, such as T lymphocytes, resulting in local resistance to the anti-inflammatory actions of CS86,89. GR isoforms (α and β) were originally described, with the nuclear GRb having a dominant negative effect on GRa through the formation of GRα/GRβ heterodimers. GRα is ubiquitously expressed in almost all human tissues and cells90 and, in the absence of ligand, resides in the cytoplasm as a heterocomplex with several shock proteins and their auxiliary molecules. In contrast to the well-known activities of GRα, the physiological role and action of GRβ are unclear. GRb is also ubiquitously expressed in almost all tissues, usually at lower concentrations than GR, with the exception of epithelial cells and neutrophils90,91. Neutrophils have a high constitutive expression of GRβ that may explain their resistance to apoptosis in response to CS and provide a mechanism for the ineffectiveness of glucocorticoid in clearing airway neutrophilia.
Most transfection studies revealed that GRβ acts as a natural dominant negative inhibitor of GR-induced transactivation of glucocorticoid-responsive genes92,93. Recent evidence in bronchoalveolar lavage fluid macrophages obtained from patients with CS resistant asthma shows increased expression of GRβ mRNA94, probably due to the action of several pro-inflammatory cytokines95. Fruchter et al showed that different synthetic CS have different susceptibility to GRβ transdominant negative activity96. They found that methylprednisolone was less affected by GRβ transdominant negative effect, compared with other steroids, a finding that may affect clinical decision-making in selecting a therapeutic derivative.
GR nuclear translocation and GR-GRE binding
The mechanism of impaired nuclear localization of the GR in response to high concentration of CS is unclear. Changes in GR-GRE binding have been associated with excessive activation of the transcription factors in response to inflammatory stimuli87,88. AP-1 levels are altered in patients with chronic resistant asthma and increased levels of AP-1 might prevent GR function. AP-1 is comprised of variable heterodimers of jun (c-jun, jun B, and jun D) and fos (c-fos, fos B, Fra-1, and Fra-2). AP-1 is activated through the transcriptional regulation of c-fos97 do 2 and the phosphorylation of c-jun, which is the end result of the action of a cascade of kinases98 do 2 C-fos expression is increased by a wide variety of growth factors and mitogens through complex signaling pathways involving activation of mitogen-activated protein kinase and calcium-dependent mechanisms99. C-jun is phosphorylated by jun N-terminal kinase (JNK), one of a group of intracellular kinases that are also known as the serum-activated protein kinases (SAPKs). There are studies suggesting that increased levels of c-Fos and increased activation of c-Jun in patients with CS resistant asthma accounts for the increased AP-1 activity seen in vitro and probably relates to increased activation of JNK in these subjects. JNK regulates the expression and activation of both major components of AP-1100. Therefore increased JNK activity could be critical to the mechanisms of CS resistant asthma, and failure to inhibit JNK phosphorylation by CS might be a major cause for the lack of response to CS in these cases87. At present, there is no evidence for a genetic component leading to enhanced AP-1 activation in CS resistant asthma. Irrespective of whether enhanced expression of AP-1 is primary or secondary, the net result is an excessive accumulation of this critical transcription factor.
In other group of patients, nuclear localization of GRs is normal, and there is a defect in acetylation of histone 4101. This suggests that CS is not able to activate certain genes that are critical to the anti-inflammatory action of high doses of CS.
CONCLUSION
Topic CS are still the gold standard in long-term anti-inflammatory management of persistent asthma and rhinitis in children. The clinical benefits of these agents by far surpass the side effects in patients treated with a low to moderate dose. However, clinical follow-up is still essential for the early detection of side effects, especially in patients taking these drugs by nasal and pulmonary routes.
Correspondence:
Dirceu Solé
Rua Mirassol 236, apto 72
04044-010 São Paulo, SP. Brazil
Phone/Fax: + 55 11 5579 1590
E-mail: dirceus@ajato.com.br
dirceusole.dped@epm.br