Kiwifruit allergy has been responsible for a variety of clinical manifestations, ranging from mild reactions, such as localised oral symptoms, to severe systemic symptoms, such as anaphylaxis. No cases of isolated contact urticaria (ICU) due to IgE-mediated allergy to kiwifruit have been reported in the literature so far. Here we describe the first three cases of ICU due to kiwi and we hypothesise about a kiwifruit allergen not described yet.
MethodsUsing the available in vivo allergy tests, we performed a component-resolved diagnosis to detect the allergen involved. All the patients underwent prick-by-prick with raw and boiled kiwi pulp and latex glove, skin prick test with commercial extracts of kiwifruit, birch, latex, palm profilin and peach lipid transfer protein, rub test with raw and boiled kiwi and oral food challenges with the raw fruit.
ResultsWe found that, in our patients, the kiwifruit allergen responsible for ICU is thermolabile, gastrosensitive, and it does not show any of the most common kiwi-attributed cross-reactivity (latex, birch, profiling and lipid transfer protein). None of the 13 kiwifruit allergens already known shows all these features.
ConclusionsKiwifruit allergy can also occur with ICU, probably due to a native protein that is not yet identified. In this case the elimination diet is not required.
Symptoms induced by IgE-mediated kiwifruit allergy can range from oral allergy syndrome (OAS), which is the most common symptom, to severe systemic reactions, such as anaphylaxis.1 However, to our knowledge, no cases of isolated contact urticaria (ICU) have been reported in children. We report the first three cases of ICU due to IgE-mediated allergy to kiwifruit in three children with atopic dermatitis. We also attempted an in vivo component resolved diagnosis (CRD) using the skin prick test (SPT) tests available in the allergologist's clinical practice.
Case reportWe observed, between January 2013 and January 2014, three females, 3–5 years old, who were all suffering from atopic dermatitis (AD) on the face, particularly on the cheeks, as well as positive family history of atopy. They presented ICU of the face (wheal and erythema on perioral region, cheeks and cheekbones), without labial and eyelid angio-oedema. These symptoms appeared while they were holding in hand and eating peeled kiwifruit which inevitably came into contact with their faces. None of them presented other signs or symptoms; particularly, no symptoms concerning the oral cavity were referred, not even concerning their fingers or hands. All three children underwent the following allergological tests, all on the same day.
Skin prick testPrick-by-prick (PbP) with raw and boiled (for 10min) kiwifruit pulp and with latex glove, and SPT with commercial extracts of kiwi, birch, latex (Lofarma, Milan, Italy), palm profilin and peach lipid transfer protein (ALK Abellò, Hørsholm, Denmark) were performed on the volar surface of the forearm of all children. Histamine (Lofarma, Milan, Italy, 10mg/ml) was used as positive control; the solvent was used as negative control. SPT was performed according to international guidelines.2 A 1mm tip metallic lancet was used (ALK Abellò, Hørsholm, Denmark). Reactions were detected after 15min and the SPT was considered positive if the mean diameter of the wheal was at least 3mm larger than the negative control.
Rub testA piece of kiwifruit was rubbed on the face (lips, cheek, eyelid, and forehead). After 10min, appearance of itchy erythema and wheals were evaluated. The test was performed with both raw and boiled kiwifruit.
Oral food challengeAn open oral food challenge (OFC) was performed in hospital. The three girls ate every 20min increasing doses of raw kiwifruit up to a total intake of two kiwifruit (the last dose was a whole kiwifruit). Food challenge was conducted making sure that kiwi and its juice did not come into contact with perioral skin. Patients stayed under clinical observation for two hours after the last dose. OFC was stopped and rated positive in case of objective symptoms and/or serious and/or persistent and/or reproducible ones.3
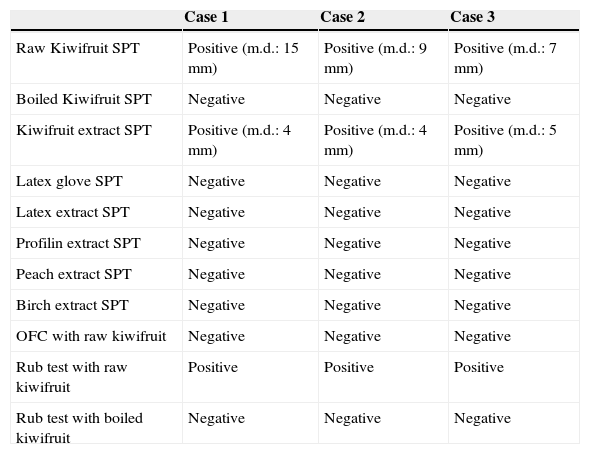
ResultsAllergy tests results are synthesised in Table 1. In summary, we observed that: (a) no adverse reactions were reported after raw kiwifruit ingestion; (b) itchy erythema and wheals appeared on the eczematous skin areas where rub test with raw kiwifruit was performed; c) rub test with boiled kiwifruit was negative; (d) only SPT with raw kiwifruit and commercial kiwi extract resulted positive.
In vivo allergy tests.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Raw Kiwifruit SPT | Positive (m.d.: 15mm) | Positive (m.d.: 9mm) | Positive (m.d.: 7mm) |
| Boiled Kiwifruit SPT | Negative | Negative | Negative |
| Kiwifruit extract SPT | Positive (m.d.: 4mm) | Positive (m.d.: 4mm) | Positive (m.d.: 5mm) |
| Latex glove SPT | Negative | Negative | Negative |
| Latex extract SPT | Negative | Negative | Negative |
| Profilin extract SPT | Negative | Negative | Negative |
| Peach extract SPT | Negative | Negative | Negative |
| Birch extract SPT | Negative | Negative | Negative |
| OFC with raw kiwifruit | Negative | Negative | Negative |
| Rub test with raw kiwifruit | Positive | Positive | Positive |
| Rub test with boiled kiwifruit | Negative | Negative | Negative |
m.d.=mean diameter; SPT=skin prick test; OFC=oral food challenge.
In case 2, a further rub test with raw kiwifruit, performed after four days of application of steroid cream on eczematous cheeks (with normalisation of the skin), showed no reaction, while itchy erythema and wheals appeared after rub test on eczematous skin of temples, where steroid cream was not applied. Rub test with raw kiwifruit on lips and eyelids, where no eczema was reported, was always negative.
DiscussionWe present the first three paediatric cases of ICU from IgE-mediated allergy to kiwifruit. Kiwifruit allergy has been observed to be responsible for a variety of clinical manifestations, ranging from mild reactions, such as OAS, to severe systemic symptoms, such as anaphylaxis, through moderate reactions, such as abdominal pain, vomiting, and generalised urticaria.1 Differences in clinical presentation of kiwifruit allergy may depend on the allergen protein involved in the IgE-mediated reaction,4 so that systemic reactors respond to more stable epitopes, whereas patients with OAS respond to unstable allergens.
The protein involved in our three cases has the following features: (a) it is probably gastrosensitive, indeed it is already destroyed by salivary enzymes, not being able to cause OAS; (b) it is thermolabile, as demonstrated by the negativity of PbP and of the Rub test with boiled kiwifruit; (c) it does not show any of the most common kiwifruit-attributed cross-reactivity (latex, birch, profiling and LTP); (d) its damaging activity is carried out only on eczematous skin areas; (e) it is probably a genuine kiwifruit allergen, because the SPT with commercial kiwi extract was always positive.4 Therefore, we have attempted to identify the protein involved among the already known kiwi allergens. We can exclude Act d1 (Actinidin), Act d 2 (Thaumatin-like protein) and Act d 5 (Kiwellin) because they are stable to heating and gastric digestion, while in our cases we never observed neither OAS nor other gastrointestinal symptoms.5 Act d 3, Act d 4 (Cystatin) and Act d 6 (Pectin metylesterase inhibitor) are associated with systemic reactions,5–7 so they are more stable than our allergen. Furthermore, Act d 3 has cross-reactivity with birch, grass pollen and latex.6 Act d 7 (Pectin metylesterase), Act d 8 (PR-10), Act d 9 (Profillin), Act d 10 (Lipid Transfer Protein) and Act d 11 (Major Latex Protein) are not specific kiwifruit allergens, cross reacting with birch pollen, profilin, peach LTP and latex.4,8 Also Act d chitinase and UDP glucose pyrophosphorylase are involved in the latex-kiwi cross-reactivity, contributing to the so called “latex-fruit allergy syndrome”.4,9 Act d 12 and Act d 13, two allergens from kiwifruit seeds recently described,10 can be excluded because they were not detected in kiwifruit extract.
In summary, none of the allergenic proteins known fully respond to all the required features. Therefore, it could be hypothesised that it is probably a protein not yet identified. Unfortunately, we have not been able to carry out immunoblotting experiments to characterise the hypothesised new allergen, because of technical difficulties.
It is probable that AD plays an important role both in pathogenesis of sensitisation as well as clinical expression, as it has been suggested for the allergen of hen's egg11: in fact, in our cases ICU was limited to the areas of skin eczema. This phenomenon may be a result of epidermal skin barrier defects. These defects can be based on genetic factors such as filaggrin mutations, other mutations in the epidermal differentiation complex located on chromosome 1q21, or variations in the expression due to the cytokine milieu.12 In animal models, it has been shown that antigens penetrated the filaggrin-null mice stratum corneum more efficiently, leading to enhanced responses in hapten-induced contact hypersensitivity and higher serum levels of anti-ovalbumin IgG1 and IgE.13 Clinical data support the findings from animal models. In a cohort study of 13,971 preschool children, Lack et al.14 observed that application of peanut oil containing creams increased the risk of developing an allergy to peanut.
Finally, our observation is relevant in order to avoid unnecessary elimination from the diet.
Conflict of interestNone.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Patients’ data protectionThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.