Common variable immunodeficiency (CVID) is a heterogeneous disease, characterised by hypogammaglobulinaemia leading to recurrent infections and various complications. The aim of this study was to classify CVID patients based on four known classifications (Paris, Freiburg, EUROclass, and B-cell patterns) by measurement of B-cell subsets and to assess the relation of each classification with clinical manifestations.
MethodsWe measured all B-cell subsets as both absolute count and percentage in 30 CVID patients and 30 healthy individuals using four-colour flow cytometry. Moreover, we evaluated antibody responses to pneumococcal vaccine in patients.
ResultsA significant reduction in percentage of terminal B-cell subsets (total, marginal zone-like, switched memory, IgM-only memory, total memory B-cells and plasmablast) and absolute count of all B-cell subsets along with a strong increase in CD21low B-cells has been observed in patients. Patients with splenomegaly and hepatomegaly clustered in group Ia, smB+21low and group 1 based on known classifications, and significantly tended to have a decreased transitional and marginal zone-like B-cells count, as well as an increase in CD21low B-cell counts. Patients with lymphadenopathy, bronchiectasis and allergy had a significant decrease in absolute count of total memory, switched memory and total B-cells, respectively.
ConclusionClassification of patients could provide useful information to guide clinicians in long-term follow-up of CVID patients. Our data demonstrate that it may be more accurate to use absolute counts of B-cell subpopulations in CVID patients because absolute counts of B-cell subsets are more associated with clinical manifestations compared with their percentage and also four known classifications.
Common variable immunodeficiency (CVID) is the second common antibody-based primary immunodeficiency, with a prevalence of about 1 in 25000 and 10545 diagnosed patient worldwide.1,2 It is associated with recurrent pyogenic infections (especially Streptococcus pneumonia and Haemophilus influenza), gastrointestinal disease, lymphoproliferative disorders, autoimmune phenomena and lymphoma.3–7 Several mutations have been identified in CVID-like patients, including inducible costimulator (ICOS), Transmembrane activator and CAML interactor (TACI), B-cell activating factor receptor (BAFF-R), CD19, CD20, CD81, CD21, lipopolysaccharide-responsive beige-like anchor (LRBA), and Ras-related C3 botulinum toxin substrate 2 (RAC2).8–13 Moreover, multiple molecular and cellular defects have been reported in CVID patients14–21; however the exact aetiology of the disease remains unknown.
Since CVID is considered as a heterogeneous group of primary antibody immunodeficiencies with various clinical and immunological features, an appropriate classification for these patients is essential. In recent years, attempts at classifying the disease based on phenotyping B-cell populations have been proposed. In 2002, Warnatz et al.22 recommended the Freiburg classification and classified CVID patients into two major groups I and II based on switched memory B-cell populations and two subgroups (Ia and Ib) based on CD21low B-cell numbers. Later, the Paris classification was proposed to categorise CVID patients into three groups (MB0, MB1, and MB2) based on only memory B-cell populations.23 According to the Paris classification, autoimmunity complications enhance in patients categorised as MB0 and MB1 groups more than to those in the MB2 group, whereas patients in the MB0 group demonstrated increased granulomatous disease and splenomegaly. Wehr et al.24 established a comprehensive classification (EUROclass) that combines the Freiburg and Paris classifications. According to EUROclass, in addition to switched memory and CD21low B-cells, patients are subdivided based on transitional B-cell populations. This classification connects various B-cell subsets with splenomegaly, granulomatous disease, and autoimmunity in patients. Recently, Driessen et al.25 offered a classification based on B-cell subset abnormalities and categorised patients into five different patterns based on the B-cell maturation arrest (pattern 1–5). Based on this classification, only splenomegaly was associated with B-cell pattern 1 in patients.
The aim of the present study was to compare various classifications (Freiburg, Paris, EUROclass and B-cell patterns) for CVID patients and to determine association of each classification with their clinical and immunological data. Moreover, we measured specific antibodies against whole pneumococcal antigens to determine whether there is an association between various responses to vaccination and the classifications in patients.
Materials and methodsPatientsWe studied a total of 30 available patients (20 males and 10 females) and 30 controls (age and sex-matched), who were referred to the Children's Medical Center (Pediatrics Center of Excellence affiliated to Tehran University of Medical Sciences, Tehran, Iran). All patients were diagnosed as CVID based on the diagnostic criteria of Pan American Group for Immune Deficiency (PAGID) criteria and the European Society for Immunodeficiencies (ESID),26 including marked decrease of IgG and marked decrease of IgA with or without low IgM levels (at least two standard deviations [SDs] below the mean for age), the exclusion of defined causes of hypogammaglobulinaemia in patients with age >4 years, poor antibody response to vaccines or low switched memory B-cells (<70% of age-related normal value) and no evidence of profound T-cell deficiency. Patients with normal response to vaccine had low switched memory B-cells and had no isohaemagglutinins; thus these patients were also considered as CVID based on ESID criteria. The research was approved by the Ethics Committee of Tehran University of Medical Sciences and Health Services and written informed consents were also obtained from all participants.
Flow cytometric analysisWhole blood was stained for 20min at 4°C with a mixture of the following antibodies at optimal concentrations: Anti-CD19 Allophycocyanin (APC), Anti-CD27 fluorescein isothiocyanate (FITC), Anti-CD21 phycoerythrin (PE), Anti-IgD (PE), Anti-CD38 (FITC), Anti-IgM peridinin chlorophyll protein (PerCp)-eFluor710 (all from eBioscience, San Diego CA, USA). All isotype controls were purchased from eBioscience and were embedded to detect unspecific staining. Using panel B1 from supplemental Table 1, B-cells were subdivided into several subpopulations: naive B-cells (CD19+CD27−IgM+IgD+), marginal zone-like B-cells (CD19+CD27+IgM++IgD+), switched memory B-cells (CD19+CD27+IgM−IgD−), and IgM-only memory B-cells (CD19+CD27+IgM++IgD−). Staining B-cells with panel B2 from supplemental Table 1 distinguished transitional B-cells (CD19+CD21intCD38++IgM++), CD21low expressing B-cell (CD19+CD21lowCD38lowIgM+) and plasmablasts (CD19lowCD21intCD38+++IgM− (+)). Finally, data were analysed by FACS Calibour instrument and CellQuest pro software (BD Biosciences, San Jose, CA, USA).
Various CVID classificationsWe classified patients based on four classifications that are explained below.
Freiburg classification: CVID patients are classified into two groups: I (switched memory B-cells below 0.4%) and II (normal numbers of switched memory B-cells). Type I patients were further subdivided into group Ia with percentages of CD21low B-cells >20% and group Ib with percentages of CD21low B-cells <20%.
Paris classification: CVID patients with a reduced percentage of CD27+ B-cells under 11% were categorised as MB0, while MB1 is related to patients with decreased class-switched memory B-cells below 8% and increased total CD27+ B-cell more than 11% of B-cells. Patients who are neither MB0 nor MB1 are classified as MB2.
EUROclass classification: EUROclass distinguishes patients with ≤1% B-cells (group B−) from patients with >1% B-cells (group B+). Patients in group B+ are divided into group smB− with ≤2% switched memory B-cells and group smB+ with >2% switched memory B-cells. SmB-patients with greater than or equal to 9% of transitional B-cells are characterised as smB-Trhi, while these patients with less than 9% of transitional B-cells are identified as smB-Trnorm. EUROClass also discriminates patients based on the expansion of CD21low B-cells above or below 10% within CD19+ B-cells (CD21low vs. CD21norm, respectively).
B-cell patterns classification: Patients with CVID could be divided into five distinct B-cell patterns based on B-cell subsets. Patients with pattern 1 demonstrate decreased numbers of transitional B-cells in combination with a reduction of memory B-cells. In B-cell pattern 2, patients show reduced transitional B-cells numbers plus reduced naive mature, marginal zone-like and memory B-cells. Patients with pattern 3 represent a reduction of both marginal zone-like and memory B-cells while patients with pattern 4 have only decreased memory B-cells. Patients have normal marginal zone-like and memory B-cells in pattern 5, while they have a reduction in plasmablasts numbers.
VaccinationA single dose of 0.5mL unconjugated pneumococcus polyvalent vaccine (PNEUMO 23® Aventis, Pasteur, France) injected intramuscularly to patients. All blood samples were taken before vaccination and 21 days after vaccination. Specific antibodies against whole pneumococcal antigens were measured using ELISA method. The method of assessment of antibody responses was explained in the previous study.27
Statistical analysisStatistical analyses were performed using SPSS 16.0 software (Chicago, USA). Using Kolmogorov–Smirnov and Shapiro–Wilk tests, we estimated whether data were normally distributed. Parametric and non-parametric analyses were performed based on the finding of these tests. Prerequisite for statistical analysis within each classification was three patients in each group.
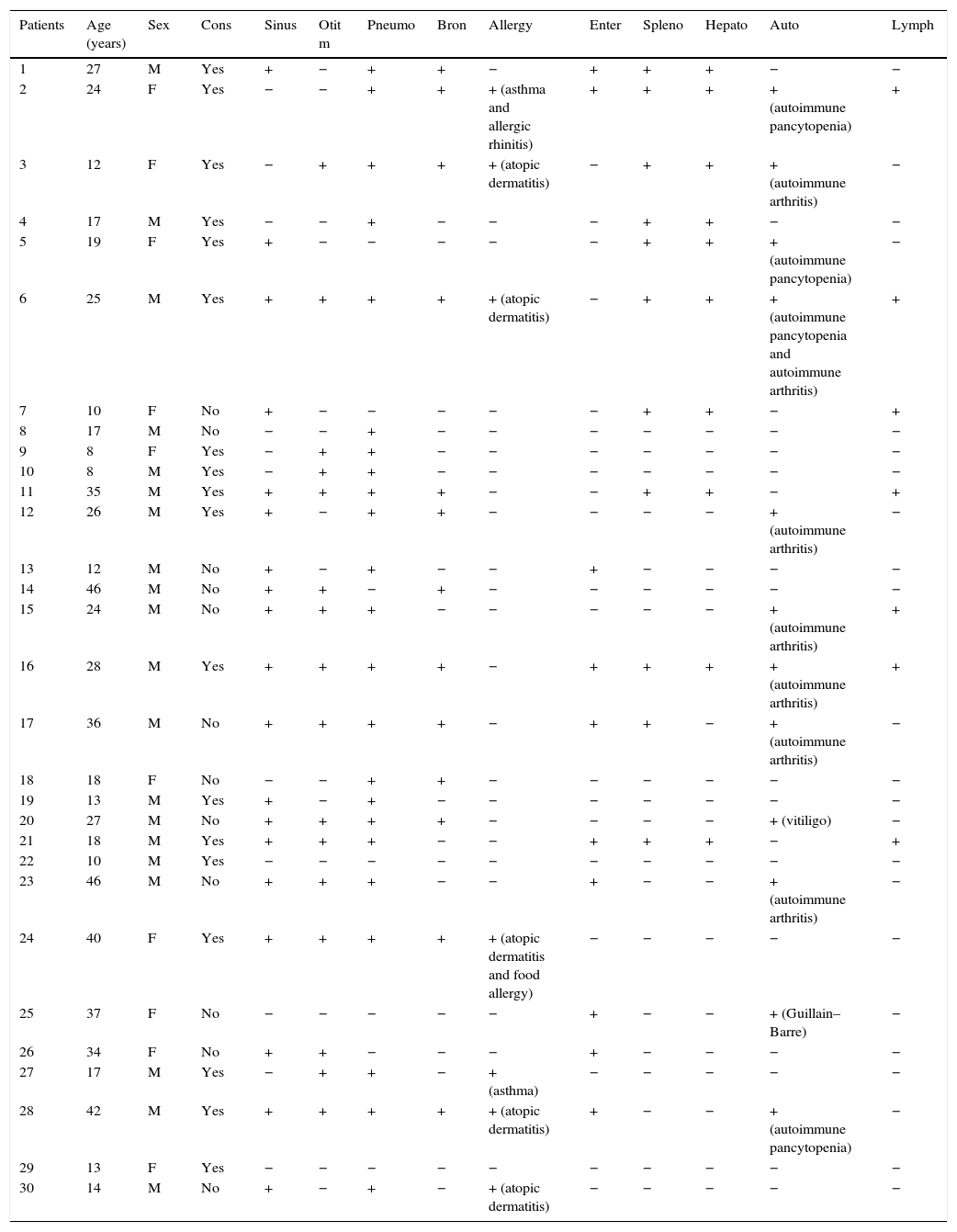
ResultsCharacteristics and clinical phenotypes of CVID patientsThe mean age of patients at the time of the study was 23.43±11.58 years. The mean age at onset of symptoms was 6.32±8.57, and the mean diagnostic delay was 6.21±5.43 years. Eighteen patients (60%) resulted from a consanguineous marriage. Demographic and immunological characteristics of patients are provided in Table 1. The most common clinical manifestations among total patients were pneumonia (23 patients), sinusitis (19 patients), otitis media (16 patients) and Bronchiectasis (13 patients). Non-infectious complications among our patients were autoimmunity (12 patients), splenomegaly (11 patients), hepatomegaly (10 patients), enteropathy (10 patients), lymphadenopathy (7 patients) and allergy (7 patients). Specific types of autoimmune and allergic diseases are demonstrated in Table 1.
Demographic and immunological data of CVID patients.
| Patients | Age (years) | Sex | Cons | Sinus | Otit m | Pneumo | Bron | Allergy | Enter | Spleno | Hepato | Auto | Lymph |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27 | M | Yes | + | − | + | + | − | + | + | + | − | − |
| 2 | 24 | F | Yes | − | − | + | + | + (asthma and allergic rhinitis) | + | + | + | + (autoimmune pancytopenia) | + |
| 3 | 12 | F | Yes | − | + | + | + | + (atopic dermatitis) | − | + | + | + (autoimmune arthritis) | − |
| 4 | 17 | M | Yes | − | − | + | − | − | − | + | + | − | − |
| 5 | 19 | F | Yes | + | − | − | − | − | − | + | + | + (autoimmune pancytopenia) | − |
| 6 | 25 | M | Yes | + | + | + | + | + (atopic dermatitis) | − | + | + | + (autoimmune pancytopenia and autoimmune arthritis) | + |
| 7 | 10 | F | No | + | − | − | − | − | − | + | + | − | + |
| 8 | 17 | M | No | − | − | + | − | − | − | − | − | − | − |
| 9 | 8 | F | Yes | − | + | + | − | − | − | − | − | − | − |
| 10 | 8 | M | Yes | − | + | + | − | − | − | − | − | − | − |
| 11 | 35 | M | Yes | + | + | + | + | − | − | + | + | − | + |
| 12 | 26 | M | Yes | + | − | + | + | − | − | − | − | + (autoimmune arthritis) | − |
| 13 | 12 | M | No | + | − | + | − | − | + | − | − | − | − |
| 14 | 46 | M | No | + | + | − | + | − | − | − | − | − | − |
| 15 | 24 | M | No | + | + | + | − | − | − | − | − | + (autoimmune arthritis) | + |
| 16 | 28 | M | Yes | + | + | + | + | − | + | + | + | + (autoimmune arthritis) | + |
| 17 | 36 | M | No | + | + | + | + | − | + | + | − | + (autoimmune arthritis) | − |
| 18 | 18 | F | No | − | − | + | + | − | − | − | − | − | − |
| 19 | 13 | M | Yes | + | − | + | − | − | − | − | − | − | − |
| 20 | 27 | M | No | + | + | + | + | − | − | − | − | + (vitiligo) | − |
| 21 | 18 | M | Yes | + | + | + | − | − | + | + | + | − | + |
| 22 | 10 | M | Yes | − | − | − | − | − | − | − | − | − | − |
| 23 | 46 | M | No | + | + | + | − | − | + | − | − | + (autoimmune arthritis) | − |
| 24 | 40 | F | Yes | + | + | + | + | + (atopic dermatitis and food allergy) | − | − | − | − | − |
| 25 | 37 | F | No | − | − | − | − | − | + | − | − | + (Guillain–Barre) | − |
| 26 | 34 | F | No | + | + | − | − | − | + | − | − | − | − |
| 27 | 17 | M | Yes | − | + | + | − | + (asthma) | − | − | − | − | − |
| 28 | 42 | M | Yes | + | + | + | + | + (atopic dermatitis) | + | − | − | + (autoimmune pancytopenia) | − |
| 29 | 13 | F | Yes | − | − | − | − | − | − | − | − | − | − |
| 30 | 14 | M | No | + | − | + | − | + (atopic dermatitis) | − | − | − | − | − |
Abbreviations: Y: years; Cons: consanguinity; Sinus: sinusitis; Otit m: otitis media; Pneumo: pneumonia; Bron: bronchiectasis; Enter: enteropathy; Spleno: splenomegaly; Hepato: hepatomegaly; Auto: autoimmunity; Lymph: lymphadenopathy.
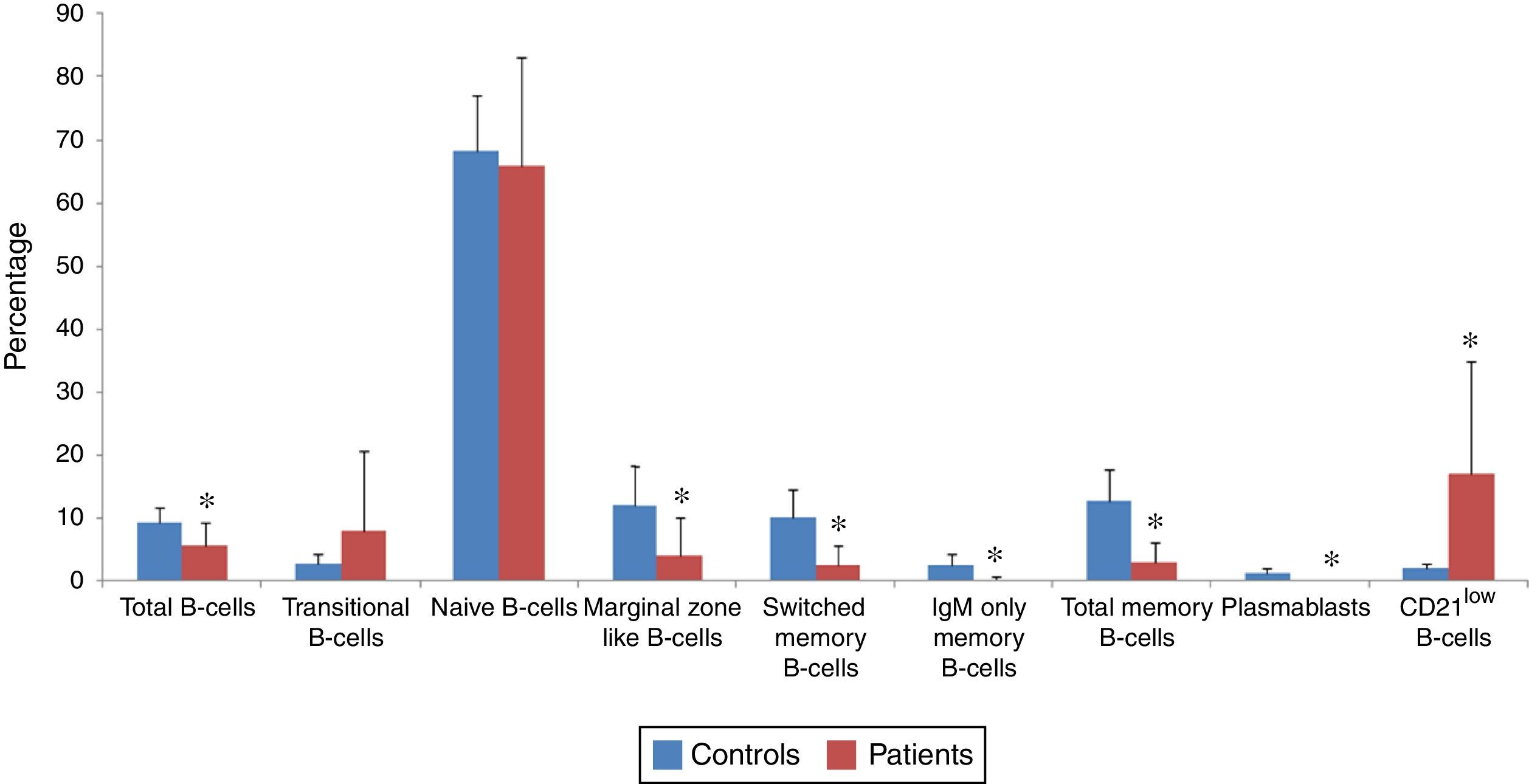
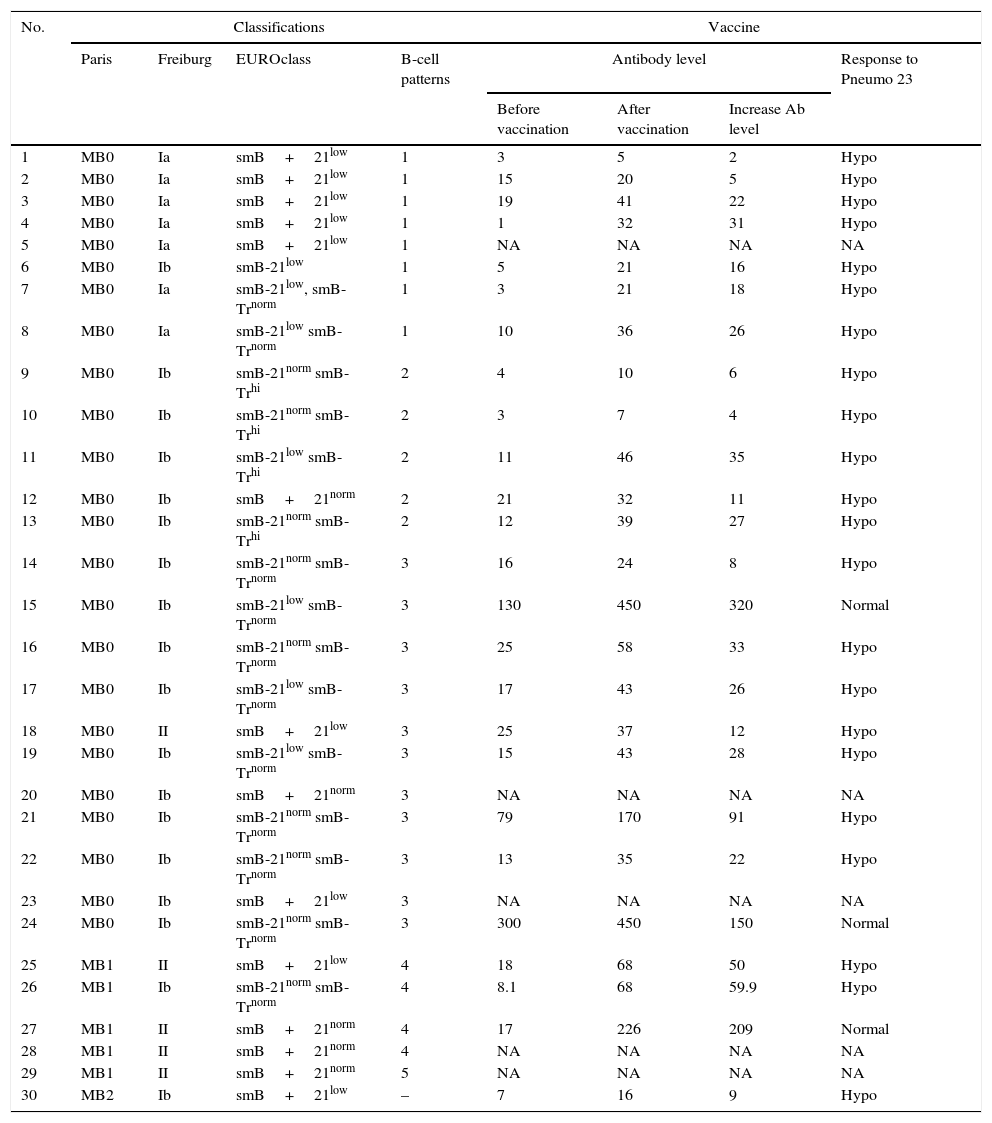
B-cell subsets of patients (n=30) and normal controls (n=30) were determined by flow cytometry (Fig. 1), and both percentage and absolute count of B-cell subsets in patients were compared with controls (Figs. 2 and 3). Regarding to the percentage of B-cell subsets, we observed a significant decrease in total (5.6±3.6% vs. 69.3±2.41%; p<0.001), marginal zone like (4.21±5.88% vs. 12.12±6.1%; p<0.001), switched memory (2.67±2.9% vs. 10.22±4.46%; p<0.001), IgM only memory (0.28±0.47% vs. 2.59±1.71%; p<0.001) and total memory (2.96±3.16% vs. 12.81±5.05%; p<0.001) B-cells as well as plasmablasts (0.01±0.09% vs. 1.28±0.7%; p<0.001) in patients compared with normal controls. On the other hand, the percentage of CD21low B-cells in patients (16.87±17.96%) was significantly higher than controls (2.09±0.77%; p<0.001). However, increased transitional B-cells (8.15±12.6% vs. 2.91±1.5%; p=0.86) and decreased naive B-cells (65.73±17.33% vs. 68.19±8.87%; p=0.49) in patients in comparison with controls were not significant.
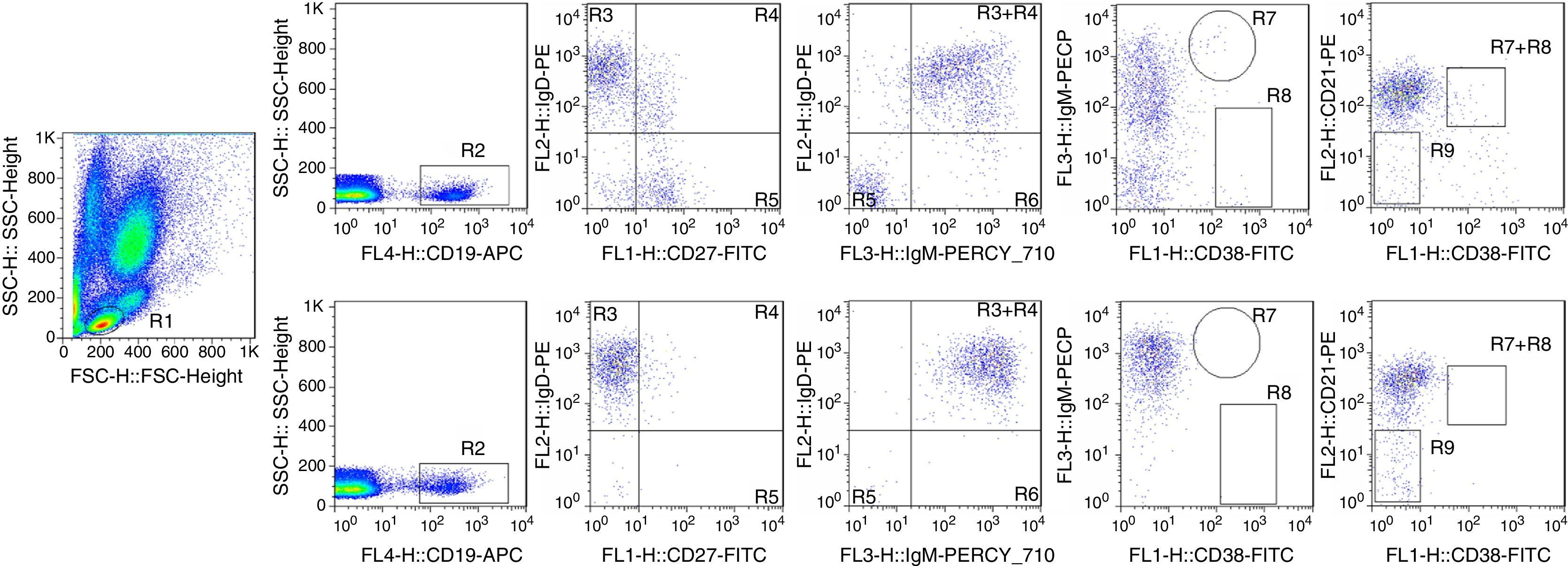
Analysis of B-cell subsets in control and CVID patients. Two examples demonstrate the flow cytometric analysis of peripheral blood samples from one control (top panels) and on the patient (down panels). CD19+ B-cells (R2) are gated within the lymphocyte scatter region (R1). Naive B-cells (R3), marginal zone-like B-cells (R4), switched memory B-cells (R5), and IgM-only memory B-cells (R6) are defined within the IgM lymphoma based on expression of CD27 and IgD. Also, transitional B-cells (R7), plasmablasts (R8) and CD21low B-cells (R9) are defined within the CD21 lymphogate based on expression of CD38 and IgM.
Flow cytometry analyses of B-cell subset percentages in CVID patients and controls. The mean percentage of B-cell subsets in CVID patients (red bars) and in controls (blue bars) are represented by Bar Charts. There was a significant reduction in the percentage of total, marginal zone-like, switched memory, IgM-only memory, total memory and CD21low B-cells as well as plasmablasts in patients compared with controls. However, elevation of transitional B-cells and reduction of naive B-cells in patients was not significant. *p<0.05, statistical significance between patients and controls.
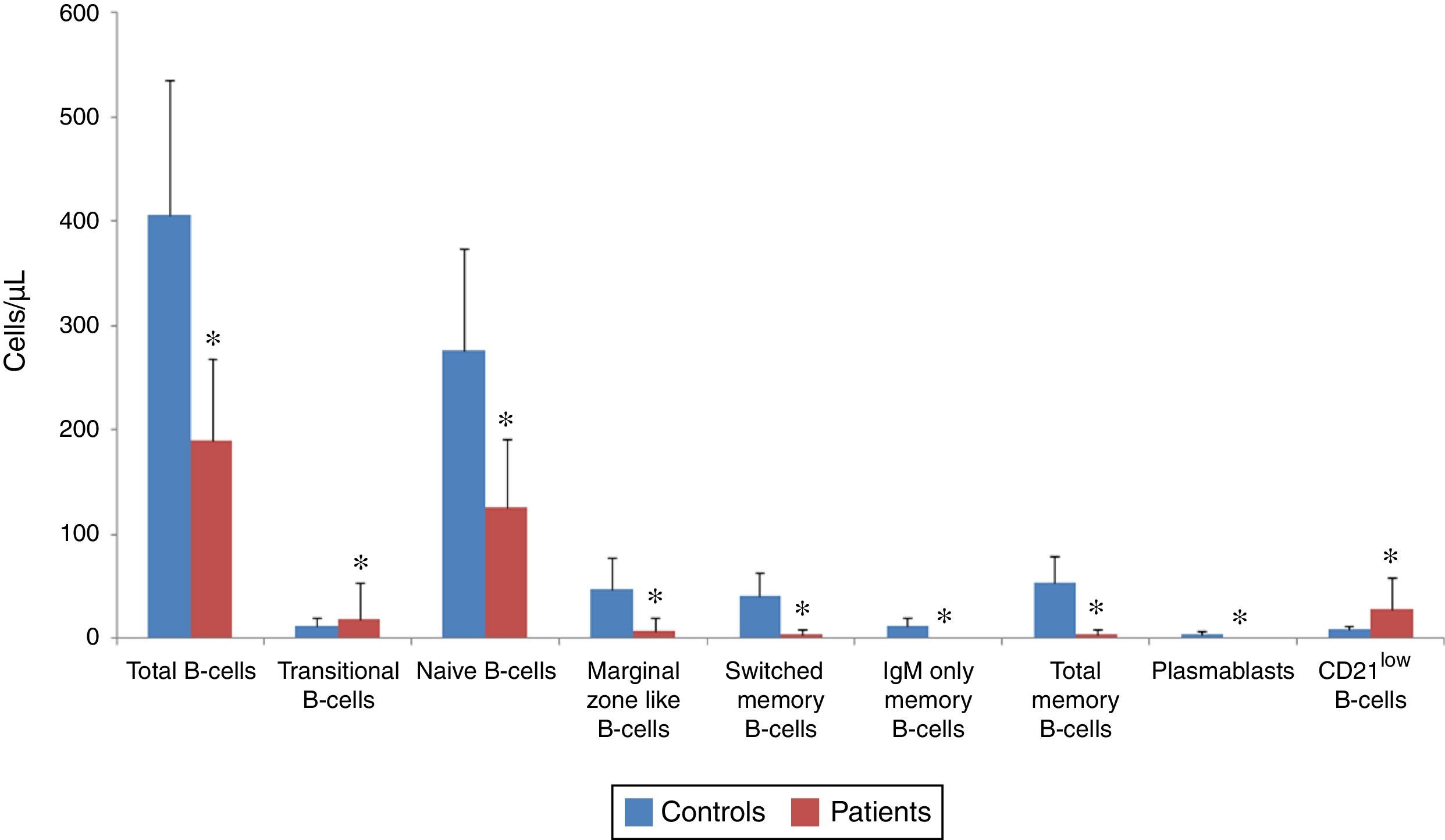
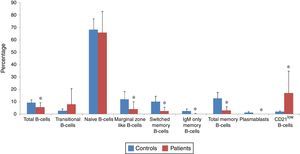
Analysis of B-cell subsets in controls and patients as an absolute count. The mean absolute count of B-cell subsets in CVID patients (red bars) and in controls (blue bars) are represented by Bar Charts. There was a significant reduction in total B-cells subsets and plasmablasts in patients compared with controls. *p<0.05, statistical significance between patients and controls.
The absolute counts of various B-cell subsets demonstrated a significant reduction in total (190.2±77.83/μL vs. 406.5±128.8/μL; p<0.001), transitional (18.96±34.24/μL vs. 12.6±7.64/μL; p=0.04), naive (126.23±66/μL vs. 275.93±98.45/μL; p<0.001), marginal zone like (8.16±12.6/μL vs. 47.46±29.77/μL; p<0.001), switched memory (4.53±4.5/μL vs. 41.6±22.23/μL; p<0.001), IgM only memory (0.36±0.66/μL vs. 11.8 vs. 9.2/μL; p<0.001) and total memory B-cells (4.9±4.9/μL vs. 53.4±25.41/μL; p<0.001) as well as plasmablasts (0.03±0.18/μL vs. 5.06±2.98/μL; p<0.001), while the absolute count of CD21low B-cells was markedly higher in patients (27.76±30.74/μL) compared with normal controls (8.66±4.2/μL; p=0.004).
Various classificationsWe classified our patients into various subgroups according to the Freiburg, Paris, EUROclass, and B-cell patterns classifications (Table 2). According to the Paris classification, 24 patients (80%) classified to MB0, while five patients (16.6%) and one patient (3.3%) had MB1 and MB2 phenotype, respectively. We recognised seven patients (23.3%) belonging to Ia group; while 18 patients (60%) were related to Ib group based on the Freiburg classification. In line with this classification, five patients (16.6%) were categorised as II group. As could be seen in Table 1, splenomegaly was identified in six patients in group Ia and five patients in group Ib, while none of the patients in group II have demonstrated splenomegaly. Moreover, hepatomegaly was seen in six patients in group Ia and four patients in group Ib, but absent in patients with group II schema. Our data demonstrated that splenomegaly and hepatomegaly significantly clustered in patients in group Ia (p=0.003 and p=0.006, respectively). Clustering of other complications was not statistically significant in different subgroups of the Freiburg classification.
Various classifications for CVID patients.
| No. | Classifications | Vaccine | ||||||
|---|---|---|---|---|---|---|---|---|
| Paris | Freiburg | EUROclass | B-cell patterns | Antibody level | Response to Pneumo 23 | |||
| Before vaccination | After vaccination | Increase Ab level | ||||||
| 1 | MB0 | Ia | smB+21low | 1 | 3 | 5 | 2 | Hypo |
| 2 | MB0 | Ia | smB+21low | 1 | 15 | 20 | 5 | Hypo |
| 3 | MB0 | Ia | smB+21low | 1 | 19 | 41 | 22 | Hypo |
| 4 | MB0 | Ia | smB+21low | 1 | 1 | 32 | 31 | Hypo |
| 5 | MB0 | Ia | smB+21low | 1 | NA | NA | NA | NA |
| 6 | MB0 | Ib | smB-21low | 1 | 5 | 21 | 16 | Hypo |
| 7 | MB0 | Ia | smB-21low, smB-Trnorm | 1 | 3 | 21 | 18 | Hypo |
| 8 | MB0 | Ia | smB-21low smB-Trnorm | 1 | 10 | 36 | 26 | Hypo |
| 9 | MB0 | Ib | smB-21norm smB-Trhi | 2 | 4 | 10 | 6 | Hypo |
| 10 | MB0 | Ib | smB-21norm smB-Trhi | 2 | 3 | 7 | 4 | Hypo |
| 11 | MB0 | Ib | smB-21low smB-Trhi | 2 | 11 | 46 | 35 | Hypo |
| 12 | MB0 | Ib | smB+21norm | 2 | 21 | 32 | 11 | Hypo |
| 13 | MB0 | Ib | smB-21norm smB-Trhi | 2 | 12 | 39 | 27 | Hypo |
| 14 | MB0 | Ib | smB-21norm smB-Trnorm | 3 | 16 | 24 | 8 | Hypo |
| 15 | MB0 | Ib | smB-21low smB-Trnorm | 3 | 130 | 450 | 320 | Normal |
| 16 | MB0 | Ib | smB-21norm smB-Trnorm | 3 | 25 | 58 | 33 | Hypo |
| 17 | MB0 | Ib | smB-21low smB-Trnorm | 3 | 17 | 43 | 26 | Hypo |
| 18 | MB0 | II | smB+21low | 3 | 25 | 37 | 12 | Hypo |
| 19 | MB0 | Ib | smB-21low smB-Trnorm | 3 | 15 | 43 | 28 | Hypo |
| 20 | MB0 | Ib | smB+21norm | 3 | NA | NA | NA | NA |
| 21 | MB0 | Ib | smB-21norm smB-Trnorm | 3 | 79 | 170 | 91 | Hypo |
| 22 | MB0 | Ib | smB-21norm smB-Trnorm | 3 | 13 | 35 | 22 | Hypo |
| 23 | MB0 | Ib | smB+21low | 3 | NA | NA | NA | NA |
| 24 | MB0 | Ib | smB-21norm smB-Trnorm | 3 | 300 | 450 | 150 | Normal |
| 25 | MB1 | II | smB+21low | 4 | 18 | 68 | 50 | Hypo |
| 26 | MB1 | Ib | smB-21norm smB-Trnorm | 4 | 8.1 | 68 | 59.9 | Hypo |
| 27 | MB1 | II | smB+21norm | 4 | 17 | 226 | 209 | Normal |
| 28 | MB1 | II | smB+21norm | 4 | NA | NA | NA | NA |
| 29 | MB1 | II | smB+21norm | 5 | NA | NA | NA | NA |
| 30 | MB2 | Ib | smB+21low | – | 7 | 16 | 9 | Hypo |
Abbreviations: NA: not assessed; hypo: hypo responsive.
All patients had greater than 1% B-cells of total lymphocytes and categorised as B+ group based on EUROclass classification. Among patients with B+ group, 14 patients (46.6%) classified as smB+ and 16 patients (53.3%) had smB− phenotype. Further analysis with an assessment of CD21low B-cells demonstrated that nine patients (30%) fell into the smB+21low group and five patients (16.6%) located in smB+21norm group, whereas seven patients (23.3%) were categorised in the smB-21low group and nine patients (30%) in the smB-21norm group. Measurement of transitional B-cells has demonstrated that 11 patients (36.6%) had a smB-Trnorm schema, whereas four patients (13.3%) had expanded transitional B-cells and fell into the smB-Trhi group. The incidence of splenomegaly (p=0.03) and hepatomegaly (p=0.038) was significantly increased in smB+21low patients compared with patients with smB+21norm phenotype. Moreover, there was a significant low delay diagnosis in smB+21low patients (4.0±2.5 years) compared with smB+21norm patients (10.6±8.2 years; p=0.03).
Based on B-cell patterns classification, patients were divided to five patterns: eight patients (26.6%) in pattern 1; five patients (16.6%) in pattern 2; eleven patients (36.6%) in pattern 3; four patients (13.3%) in pattern 4 and only one patient (3.3%) in pattern 5, while one patient has located in none of the five patterns. Splenomegaly significantly clustered in patients with B-cell pattern 1 (p=0.04), however, other complications were not significantly correlated with any of the five B-cell patterns. Our data demonstrated that patients with splenomegaly and hepatomegaly significantly tend to have decreased absolute count of transitional (7.18±16.62/μL vs. 25.78±40.01/μL; p=0.01 and 7.4±17.5/μL vs. 24.75±39.22/μL; p=0.01, respectively) and marginal zone-like B-cells (1.9±2.91/μL vs. 11.78±14.96/μL; p=0.003 and 2.0±3.05/μL vs. 11.25±14.76/μL; p=0.006, respectively), as well as increased CD21low B-cell counts (47.54±37.35/μL vs. 16.31±19.24/μL; p=0.007 and 50.7±37.79/μL vs. 16.3±18.72/μL; p=0.005, respectively).
We analysed the association of splenomegaly and hepatomegaly with percentage and absolute count of B-cell subsets. We observed that reduced transitional (4.29±10.34% vs. 10.38±13.48%; p=0.01 and 4.25±10.9% vs. 10.1±13.19%; p=0.004, respectively) and marginal zone-like B-cells (0.92±1.23% vs. 6.11±6.67%; p=0.002 and 0.93±1.3% vs. 5.85±6.6%; p=0.003, respectively), as well as increased CD21low B-cell percentage (30.4±20.8% vs. 9.03±10.1%; p=0.006 and 31.84±21.37% vs. 9.38±9.95%; p=0.009, respectively) were significantly associated with splenomegaly and hepatomegaly. However, patients with splenomegaly had lower switched memory B-cells (2.81±2.44%) than patients without splenomegaly (5.52±5.14%), but this was not significant (p=0.06). The incidence of lymphadenopathy was markedly increased in patients with decreased absolute count of total memory (p=0.01) and switched memory B-cells (p=0.01). Moreover, patients with bronchiectasis and allergy had a significant reduction in the absolute count of total B-cells (158.45±54.61/μL vs. 213.12±83.9/μL; p=0.04 and 144.86±36.72/μL vs. 203.04±80.93/μL; p=0.01, respectively), whereas this difference was not significant about the percentage. Although autoimmunity was more seen in patients with expanded CD21low B-cells (31.33±38.96/μL and 21.77±20.73%) compared with normal CD21low B-cells (25.38±24.79/μL and 14.35±15.37%), this difference was not significant (p=0.84 and 0.13, respectively).
We evaluated whether difference in response to the vaccine could be associated with classifications. Evaluation of antibody response to the pneumococcal vaccine was done in 25 patients who agreed to complete our investigation. Our results indicated that 22 patients (88%) demonstrated hypo response to vaccination and three patients (12%) had an adequate response to the pneumococcal vaccine. None of the patients with normal response to vaccine had splenomegaly and hepatomegaly. Among patients with normal response to vaccine, two patients had ≤2% switched memory B-cells (smB−) and one patient >2% switched memory B-cells (smB+), two patients presented with below 10% B-cell CD21low (21norm) and one patient above 10% B-cell CD21low (CD21low). Transitional B-cell numbers were normal (Trnorm) in patients with normal response. We have not found any correlation between vaccination outcome and different classifications.
DiscussionCVID is a heterogeneous immunodeficiency disease with various complications in which B-cell defects have been shown.17 During the last few years, various efforts have been made to develop classification systems on the basis of laboratory findings correlated with clinical features to classify CVID patients into more homogenous subgroups, however further studies need to confirm an appropriate classification for patients with CVID.28 In the present study, for the first time, we classified our patients according to four known classifications including Paris, Freiburg, EUROclass and B-cell pattern classifications and evaluated the association of each classification with clinical and immunological data.
Our results indicate that there is a significant decrease in the percentage of total, marginal zone-like, switched memory, IgM-only memory and total memory B-cells as well as plasmablasts, while the difference in the percentage of earlier B-cell subsets such as transitional and naive B-cells is not significant. These data demonstrate that the defects are in the terminal stages of B-cells differentiation, as has been described in previous reports.17,29 However, the analysis of B-cell subsets as absolute count demonstrated different results compared to the percentage of these cells, as we observed a significant reduction in all B-cell subsets. Decrease in the absolute count of all B-cell subsets were identified in other studies that were consistent with our results.25,30 The discrepancy between percentage and absolute count of B-cell subsets in patients clarifies this point that measurement of B-cell subset percentage demonstrates the terminal B-cell defects while evaluation of B-cell subset counts indicates defective B-cell productions. This discrepancy between percentage and absolute count of B-cell subsets in CVID patients has been previously observed by Al Kindi et al.30 Decrease in B-cell subsets could be due to increasing in apoptosis of B-cells16 or to arrest in early B-cell development in bone marrow in CVID patients.31 Since B-cell subset counts were calculated as cells per microlitre of blood, in contrast to a percentage, the absolute count of a specific B-cell subset is not influenced by an increase or decrease of the other B-cell subsets. Furthermore, some B-cell subsets may not be measured by a particular analysis method, while this could be effective in the distribution of B-cell subsets’ percentage. However, further studies with a larger number of patients and different genetic background of other countries are needed to clarify the discrepancy between percentage and absolute count of B-cell subsets in patients. Patients with splenomegaly and hepatomegaly were associated with reduced absolute numbers and percentage of the transitional and marginal zone-like B-cells, whereas patients with lymphadenopathy tended to have a lower absolute count of total memory and switched memory B-cells. Driessen et al. reported similar findings regarding the association between splenomegaly and a decreased number of transitional B-cells in CVID patients.25 We have found a reduction in switched memory B-cells in patients with splenomegaly, but our results were not significantly similar to other studies that might be due to the relatively low number of our patients.24,32 Regarding the measurement of absolute counts of B-cell subsets, bronchiectasis, and allergy in our patients were significantly associated with decreased total B-cell counts, but this difference was not significant regarding the percentage. Newly, we reported a cohort study regarding the prevalence of asthma and allergic diseases (11.7%) in our CVID patients33 that indicate allergic diseases are almost significant among CVID patients and association of B-cell counts and allergic diseases could be useful for diagnosis and classification of these patients. To our knowledge, we have not found any report of a significant correlation between decreased B-cell with allergy and bronchiectasis, suggesting further studies about this correlation. These results demonstrate that absolute count of B-cell subsets is a more reliable method for assessment of CVID complications compared with the percentage of B-cell subsets.
CD21low B-cell is an unusual population that is not considered as a known stage of B-cell differentiation, as one study indicates this subset is an autoreactive unresponsive clone34 while other studies suggested it as a human innate-like B-cell35 or tissue-like memory B-cells.36 We found a significant increase in the percentage and absolute count of CD21low B-cells in CVID patients similar to previous studies.25,37 Consistent with previous studies,24,38,35 we observed that patients with splenomegaly demonstrate expanded percentage and absolute count of CD21low B-cell compared with the patient without splenomegaly, indicating a significant direct correlation between splenomegaly and expanded CD21low B-cells. It seems reductions of the transitional and marginal zone-like B-cells along with expanded of CD21low B-cells are proper markers for splenomegaly in CVID patients. It remains speculative whether splenomegaly precedes the reduction of the transitional and marginal zone-like B-cells along with expanded of CD21low B-cells or vice versa.
We compared our results with the four known classifications. Consistent with previous studies,24,30,38,39 we observed more patients categorised as MB0 compared with MB1 and MB2 based on the Paris classification. Since more patients are located in MB0, thus, more complications were observed in this subgroup. Comparison of complications in MB0 subgroup with MB1 and MB2 subgroups was not logical because a prerequisite for statistical analysis within each classification was at least three patients in each group. We found splenomegaly was clustered in group Ia of the Freiburg classification similar to previous reports.38,24,22 In addition to splenomegaly, we identified that hepatomegaly was also clustered in group Ia, indicating that reduction of switched memory B-cells and expansion of CD-21low B-cells in patients could be risk factors for both splenomegaly and hepatomegaly. Other complications had no significant correlation with any of these three groups of the Freiburg classification, highlighting that the Freiburg classification is a proper classification for detection of organomegaly in CVID patients. According to EUROclass classification and using specific subtyping of patients with normal counts of switched memory B-cells, we observed that lymphoproliferation was significantly higher in patients with smB+21low schema while other complications were not significantly different among subgroups. Association of smB+21low and splenomegaly has been previously demonstrated by Wehr et al.24 In keeping with this, we also found a significant correlation between patients with smB+21low schema and organomegaly. The correlation between patients with smB+Trnorm schema and organomegaly is very important because our patients demonstrated lower diagnosis delay than patients with smB+21norm and without organomegaly. When we classified our patients based on B-cell patterns, distribution of patients within different patterns was similar to those reported by Driessen et al.,25 as the highest number of patients were in groups 3, 1, 2, 4 and 5, respectively. We also found that only splenomegaly significantly clustered in patients with B-cell pattern 1, however, a significant correlation between other complications and any of the five B-cell patterns has not been identified. These findings are consistent with only one published study that classified patients based on B-cell patterns.25 Our data demonstrate that patients in group 1 who have a defect in B-cell production and germinal centre formation are more associated with clinical complications.
Regarding antibody responses to the pneumococcal vaccine, we have found that patients with normal response have not demonstrated any splenomegaly and hepatomegaly. In keeping with this, we have previously reported that the presence of splenomegaly is associated with poor response to vaccine.40,41 Although poor responses to vaccine could be associated with a defect in B-cell subset numbers in patients, we have not identified any significant correlation between antibody responses to the pneumococcal vaccine with any of the classifications. Further studies with a larger number of patients need to investigate whether there is a correlation between antibody response to vaccine with each of the classifications.
ConclusionWe classified CVID patients based on four known classifications (Paris, Freiburg, EUROclass and B-cell patterns) as well as measurement of B-cell subsets and evaluated their correlation with clinical manifestations. Based on these four known classifications, we found that only organomegaly was associated with some subgroups of these classifications. When we measured absolute count of B-cell subsets, our results indicated that B-cell subsets have different results compared to the percentage of these cells, as bronchiectasis and allergy were also significantly associated with decreased total B-cell counts, but this difference was not significant regarding the percentage. Furthermore, we observed a significant reduction in all B-cell subsets (except CD21low B-cells) as absolute count, while this reduction was only in some B-cell subsets as a percentage. These results demonstrate that measurement of B-cell subsets as absolute count is a more reliable approach for evaluation of CVID patients compared with the percentage of B-cell subsets. Indeed, we found that it may be more accurate to use absolute counts of B-cell subpopulations in future studies regarding patients with CVID because absolute counts of B-cell subsets are more associated with clinical manifestations compared with their percentage and also the four known classifications. However, further studies with a larger number of patients and different genetic backgrounds of other countries are needed to confirm our results.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestNo conflict of interest is reported for any of the authors.
This work was supported by Isfahan University of Medical Sciences (Grant #393026) and Tehran University of Medical Sciences (Grant #25535).