Sickle cell disease (SCD) children are at increased risk of invasive pneumococcal disease and rely on penicillin prophylaxis and vaccination for infection prevention. Post-vaccination antibody levels in SCD may wane overtime. HbSC are believed to have better immunological response than HbSS.
ObjectiveTo compare antibody response to 23-valent pneumococcal polysaccharide vaccine (PPSV-23) between HbSS and HbSC.
MethodsPatients with HbSS (n=33) and HbSC (n=11), aged 7–18 years, were prospectively recruited. Luminex pneumococcal antibody levels were measured for 23-serotypes, after two PPSV-23 doses.
ResultsAbsolute median titer for 20 of the 23 serotypes was higher in HbSC than HbSS and significantly higher for serotypes 22 (3.9 vs. 1.6mcg/ml; p=0.039) and 43 (2.9 vs. 0.8mcg/ml; p=0.007). HbSC mounted a better immune anti-pneumococcal response compared to HbSS (≥1.3mcg/ml) for 18 of 23 serotypes, albeit not significant for any of the serotypes. More HbSC (64%) than HbSS (42%) were good vaccine responders (p=0.303). Two of 21 (10%) good vaccine responders and nine of 23 (39%) poor vaccine responders SCD participants subsequently developed acute chest syndrome or pneumonia (p=0.036). None of the HbSC patients developed ACS after receiving PPSV-23. HbSS poor vaccine responders were at increased future recurrence risk for ACS (p=0.003), pneumonia (p=0.036) or both (p=0.011), compared to good vaccine responders.
ConclusionHbSC possess better pneumococcal vaccine response than HbSS. Poor vaccine response is concerning for future acute pulmonary events. Current vaccination strategy for SCD sub-types are lacking, therefore further study to evaluate utility of vaccine boosters is necessary.
Sickle cell disease (SCD) is the most common inherited hematologic disorder in the United States. Previous studies have established that individuals with SCD are at higher risk of infections, particularly by encapsulated bacteria such as Streptococcus pneumoniae.1 The single most common cause of death in children with SCD is Streptococcus pneumoniae sepsis.1,2 The unusual susceptibility results from two immunological problems; (1) splenic malfunction, and (2) failure to make specific IgG antibodies to polysaccharide antigens. As a result of this increased risk, children with SCD are heavily reliant on both early penicillin prophylaxis and anti-pneumococcal vaccination for prevention of invasive pneumococcal disease (IPD). The current ACIP guidelines released by the National Heart Lung and Blood Institute (NHLBI) recommend all children complete the primary PCV-13 series, and SCD children also receive the 23-valent polysaccharide anti-pneumococcal vaccine (PPSV-23) but recommendations regarding additional doses was not specified.3
Children with SCD typically do not respond to the pneumococcal vaccine as well as otherwise healthy children. Although the overall immune response is poor, a rise in IgG antibodies against the most immunogenic polysaccharides and a lower response to less immunogenic polysaccharides is often noted. IgG antibody concentrations in five-year-old children with SCD, who were previously immunized with one or two doses of the polysaccharide vaccine were found to have levels similar to those of unimmunized healthy children. Apparently there was no evidence that the polysaccharide vaccine provided a “booster” effect.4 Only 36% of SCD patients had sustained anti-pneumococcal protective antibody levels three years following vaccination.5 The less-immunogenic antigens are likely responsible for vaccine failures.
Some studies have demonstrated that it was safe to discontinue prophylactic penicillin at age five.6 Despite these results, some clinicians still continue prophylaxis beyond age five years, but this approach is less popular now that penicillin resistant organisms have emerged.7 A “just in time” approach is sometimes suggested as an alternative to prophylaxis for children with HbSC or HbSβthal+, whereupon the incidence of Streptococcus pneumoniae sepsis is lower than in sickle cell anemia (HbSS). Studies show that up to 69% of pediatric hematologists stop penicillin prophylaxis and rely on immunizations to prevent IPD after the age five of years.8
Patients with HbSS and HbSC are believed to have differing immune responses to vaccination, but a direct comparison between the two groups has not previously been performed. We hypothesized that children with HbSC had a better immune response to pneumococcal vaccination than HbSS.
Materials and methodsStudy design and participantsIn this prospective study, all patients aged 18 and below having a confirmed diagnosis of SCD with hemoglobin subtypes HbSS and HbSC seen at our institute during a maintenance visit at a steady state with no acute pain crisis, hospitalization, fever or blood transfusion in the previous four weeks, were included. The comprehensive sickle cell center is a major urban tertiary referral center and medical home for sickle cell patients in the region, providing multidisciplinary care to our patients, including hematology, primary care, pulmonary, psychiatry, social worker and nurse coordinator. Subjects had to have undergone vaccination response testing at least four weeks after receiving two doses of PPSV-23 (Pneumovax®) vaccines. Per guidelines at our institute, all SCD patients receive one dose of PPSV-23 of 0.5ml between the ages of two to five years, followed by a second dose of 0.5ml five years thereafter; as a preventive measure given the high risk of invasive pneumococcal disease among the SCD patients. We did not find any children younger than age seven years who had received two doses of PPSV. The study was approved by the institutional review board (IRB number: HSC-MS-17-0937).
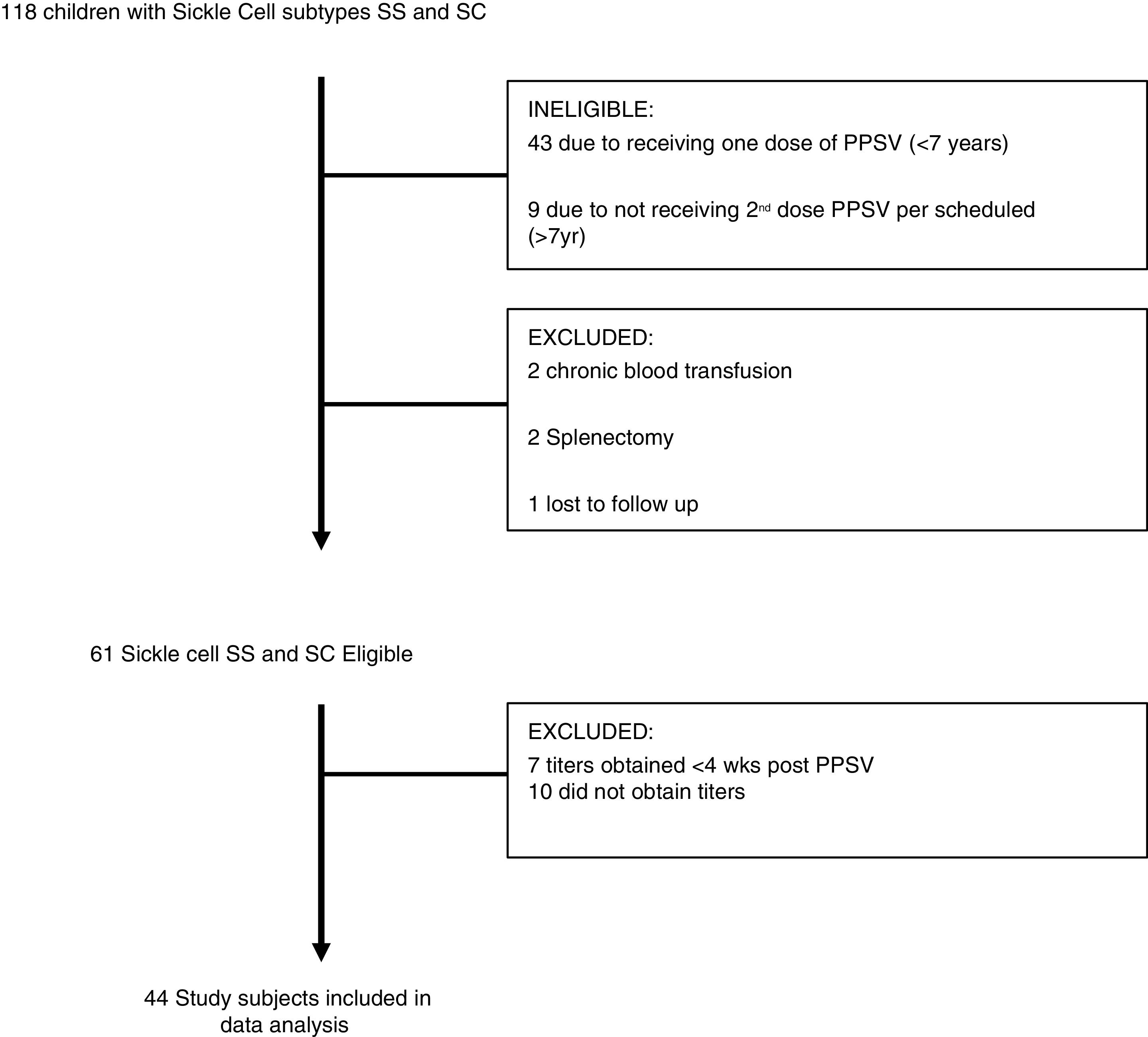
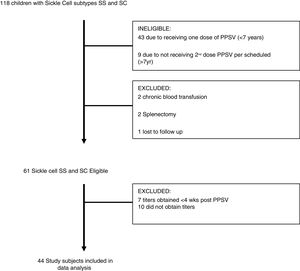
Subjects would also have received PCV (pneumococcal conjugate vaccine) based on vaccination schedule. Subjects with unknown history of immunization, fewer than two doses of PPSV, unknown levels of pneumococcal antibody titers, history of chronic blood transfusion or recent blood transfusion within the past four weeks and history of known immunodeficiency, including splenectomy were excluded from the study. Demographic data such as age, gender, and SCD subtype, immunization history and anti-pneumococcal immunoglobulin titers done in commercial laboratory, documentation of acute chest syndrome (ACS) or pneumonia in the medical record were obtained. The tested serotypes titers (American nomenclature) correspond to those covered by PPSV-23 (Pneumovax®), including 1, 2, 3, 4, 5, 8, 9, 12, 14, 17, 19, 20, 22, 23, 26, 34, 43, 51, 54, 56, 57, 68 and 70. Of the 118 children seen at out institute 51 were ineligible due to receiving only one dose of PPSV and five were excluded due to history of splenectomy (n=2), chronic blood transfusions (n=2) and lost to follow up (n=1). Forty-four eligible subjects (72%) eventually completed the study. (Fig. 1)
Vaccine response assessmentThe vaccination response testing was performed as part of the routine comprehensive assessment at our center. Immunoglobulin levels were measured using Luminex multiple analyte profiling system, using quantitative multiplex bead assay done by commercial labs.9,10 Luminex multiple analyte profiling involves a flow cytometric system that allows for single sample testing against multiple analytes. This technique utilizes competitive inhibition binding at various dilutions with 1-h incubation periods in order to assess serum immunogenicity against a wide array of pneumococcal serotypes. An individual patient's immune response is defined both per serotype as well as the overall vaccination response to all serotypes tested.
Interpretation of pneumococcal vaccine responsePatients are considered to be “good vaccine responders” if their serum IgG levels were ≥1.3mcg/ml to at least 70% of the 23 pneumococcal serotypes tested (i.e. more than 16 serotypes with levels of greater than or equal to 1.3mcg/ml) based on previous reports.11,12 Patients who responded to 15 or less of the designated serotypes were defined as “poor vaccine responders”.
Statistical analysisAnalyses were conducted using Stata statistical software (v14, StataCorp LP, College Station, TX, USA). Categorical data are reported as frequencies (with percentages) and comparisons across categorical variables were performed using chi-square of Fisher exact test. Continuous data was described using means (with standard deviations, SD) and medians (with interquartile ranges, IQR) for data that was normally and not normally distributed, respectively. The distribution of these variables was compared between the SS and SC patient groups using a t-test (if normally distributed) or a Mann–Whitney test (if not normally distributed).
ResultsPneumococcal vaccination titers were collected on all patients seen at our pediatric sickle cell center who had been vaccinated with PPSV-23. Of the 118 children, 61 subjects were eligible although 44 (72%) eventually completed the study (Fig. 1). Of these, 21 were male (48%), 33 HbSS disease (75%), 11 HbSC (25%) and 28 (HbSS-27/33) on hydroxyurea (Table 1). The mean age of all subjects was 12.7 years, with a range of 7–18 years.
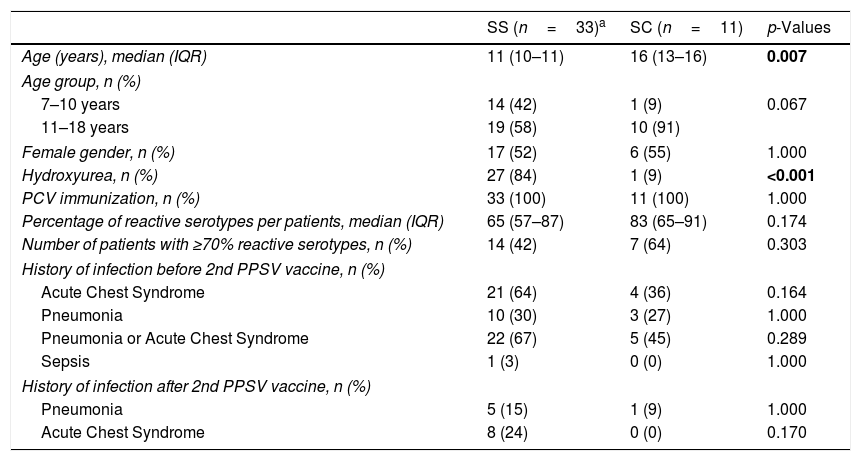
Comparison of demographic and medical history between HbSS and HbSC patients.
| SS (n=33)a | SC (n=11) | p-Values | |
|---|---|---|---|
| Age (years), median (IQR) | 11 (10–11) | 16 (13–16) | 0.007 |
| Age group, n (%) | |||
| 7–10 years | 14 (42) | 1 (9) | 0.067 |
| 11–18 years | 19 (58) | 10 (91) | |
| Female gender, n (%) | 17 (52) | 6 (55) | 1.000 |
| Hydroxyurea, n (%) | 27 (84) | 1 (9) | <0.001 |
| PCV immunization, n (%) | 33 (100) | 11 (100) | 1.000 |
| Percentage of reactive serotypes per patients, median (IQR) | 65 (57–87) | 83 (65–91) | 0.174 |
| Number of patients with ≥70% reactive serotypes, n (%) | 14 (42) | 7 (64) | 0.303 |
| History of infection before 2nd PPSV vaccine, n (%) | |||
| Acute Chest Syndrome | 21 (64) | 4 (36) | 0.164 |
| Pneumonia | 10 (30) | 3 (27) | 1.000 |
| Pneumonia or Acute Chest Syndrome | 22 (67) | 5 (45) | 0.289 |
| Sepsis | 1 (3) | 0 (0) | 1.000 |
| History of infection after 2nd PPSV vaccine, n (%) | |||
| Pneumonia | 5 (15) | 1 (9) | 1.000 |
| Acute Chest Syndrome | 8 (24) | 0 (0) | 0.170 |
Prior to receiving the 2nd dose PPSV-23, 27 patients (22 HbSS and five HbSC) reported history of pneumonia or acute chest syndrome and one HbSS had Streptococcus pneumoniae sepsis. There was no significant difference in occurrence of ACS, pneumonia or sepsis between both hemoglobin sub-types (Table 1). After receiving two doses of PPSV-23, 11 patients (ten HbSS and one HbSC) were subsequently treated for pneumonia or acute chest syndrome. There was no significant difference in ACS or pneumonia between the hemoglobin sub-types. Of note, SCD patients with no prior ACS did not develop any ACS episodes even after the 2nd vaccine dose. These patients were not on penicillin prophylaxis during the episodes of pneumonia or acute chest syndrome.
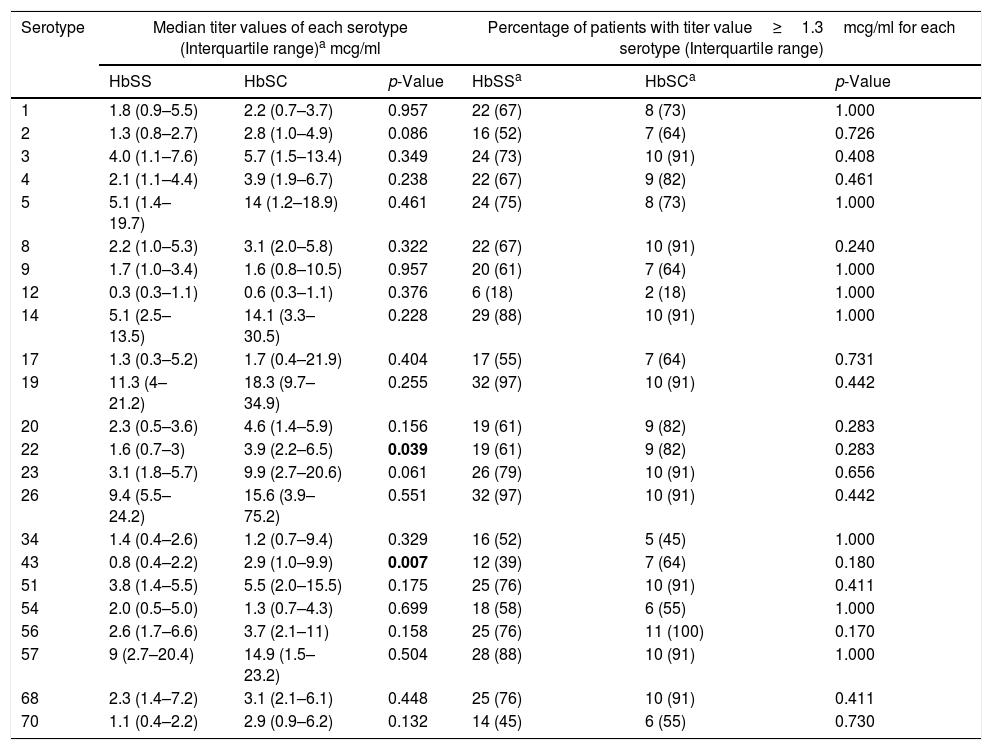
The absolute median value of the titers among patients with HbSC was higher compared to HbSS patients to all 23-serovalents, except serotypes 9, 34 and 54. However, due to the considerable variability in the titer values for each patient, a statistically significant difference in these values (Table 2) was observed only for serotypes 22 (3.9 vs. 1.6mcg/ml; p=0.039) and 43 (2.9 vs. 0.8mcg/ml; p=0.007).
Median titer values and percentage of patients with titer values ≥1.3mcg/ml for each serotype.
| Serotype | Median titer values of each serotype (Interquartile range)a mcg/ml | Percentage of patients with titer value≥1.3mcg/ml for each serotype (Interquartile range) | ||||
|---|---|---|---|---|---|---|
| HbSS | HbSC | p-Value | HbSSa | HbSCa | p-Value | |
| 1 | 1.8 (0.9–5.5) | 2.2 (0.7–3.7) | 0.957 | 22 (67) | 8 (73) | 1.000 |
| 2 | 1.3 (0.8–2.7) | 2.8 (1.0–4.9) | 0.086 | 16 (52) | 7 (64) | 0.726 |
| 3 | 4.0 (1.1–7.6) | 5.7 (1.5–13.4) | 0.349 | 24 (73) | 10 (91) | 0.408 |
| 4 | 2.1 (1.1–4.4) | 3.9 (1.9–6.7) | 0.238 | 22 (67) | 9 (82) | 0.461 |
| 5 | 5.1 (1.4–19.7) | 14 (1.2–18.9) | 0.461 | 24 (75) | 8 (73) | 1.000 |
| 8 | 2.2 (1.0–5.3) | 3.1 (2.0–5.8) | 0.322 | 22 (67) | 10 (91) | 0.240 |
| 9 | 1.7 (1.0–3.4) | 1.6 (0.8–10.5) | 0.957 | 20 (61) | 7 (64) | 1.000 |
| 12 | 0.3 (0.3–1.1) | 0.6 (0.3–1.1) | 0.376 | 6 (18) | 2 (18) | 1.000 |
| 14 | 5.1 (2.5–13.5) | 14.1 (3.3–30.5) | 0.228 | 29 (88) | 10 (91) | 1.000 |
| 17 | 1.3 (0.3–5.2) | 1.7 (0.4–21.9) | 0.404 | 17 (55) | 7 (64) | 0.731 |
| 19 | 11.3 (4–21.2) | 18.3 (9.7–34.9) | 0.255 | 32 (97) | 10 (91) | 0.442 |
| 20 | 2.3 (0.5–3.6) | 4.6 (1.4–5.9) | 0.156 | 19 (61) | 9 (82) | 0.283 |
| 22 | 1.6 (0.7–3) | 3.9 (2.2–6.5) | 0.039 | 19 (61) | 9 (82) | 0.283 |
| 23 | 3.1 (1.8–5.7) | 9.9 (2.7–20.6) | 0.061 | 26 (79) | 10 (91) | 0.656 |
| 26 | 9.4 (5.5–24.2) | 15.6 (3.9–75.2) | 0.551 | 32 (97) | 10 (91) | 0.442 |
| 34 | 1.4 (0.4–2.6) | 1.2 (0.7–9.4) | 0.329 | 16 (52) | 5 (45) | 1.000 |
| 43 | 0.8 (0.4–2.2) | 2.9 (1.0–9.9) | 0.007 | 12 (39) | 7 (64) | 0.180 |
| 51 | 3.8 (1.4–5.5) | 5.5 (2.0–15.5) | 0.175 | 25 (76) | 10 (91) | 0.411 |
| 54 | 2.0 (0.5–5.0) | 1.3 (0.7–4.3) | 0.699 | 18 (58) | 6 (55) | 1.000 |
| 56 | 2.6 (1.7–6.6) | 3.7 (2.1–11) | 0.158 | 25 (76) | 11 (100) | 0.170 |
| 57 | 9 (2.7–20.4) | 14.9 (1.5–23.2) | 0.504 | 28 (88) | 10 (91) | 1.000 |
| 68 | 2.3 (1.4–7.2) | 3.1 (2.1–6.1) | 0.448 | 25 (76) | 10 (91) | 0.411 |
| 70 | 1.1 (0.4–2.2) | 2.9 (0.9–6.2) | 0.132 | 14 (45) | 6 (55) | 0.730 |
Subjects with HbSC mounted a better immune response to the pneumococcal vaccination compared to HbSS (serum IgG levels≥1.3mcg/ml). HbSC patients had 21 serotypes that were≥1.3mcg/ml, compared to 20 serotypes for HbSS. Percentage of HbSC mounting an adequate response, was equal to or higher than HbSS for 18 of the 23 serotypes, but there was no statistical difference between the groups (Table 2).
Patients with HbSC showed higher reactivity (serum IgG levels≥1.3mcg/ml to at least 70% of the 23 serotypes) than HbSS patients (Table 1). More HbSC patients 64% (n=7) were “good vaccine responders”, compared to 42% (n=14) HbSS patients (p=0.303).
In SCD patients post-vaccination, two of the 21 (10%) “good vaccine responders”, and nine of 23 (39%) “poor vaccine responders” subsequently went on to develop ACS or pneumonia (p=0.036). “Poor vaccine responders” (n=8, 100%) were significantly more likely than “good vaccine responders” to develop ACS (p=0.004) and more “poor vaccine responders” (n=4; 42%) than “good vaccine responders” (n=2; 10%) developed pneumonia (p=0.423). The occurrence of ACS, pneumonia, or both were not significantly different (Table 1) between both hemoglobin sub-types. Of note, all ACS after 2nd PPSV-23 (n=8) were HbSS, all of whom were “poor vaccine responders”. When vaccine response was assessed by hemoglobin sub-type to infection risk, HbSS “poor vaccine responders” (n=19) with a prior history of ACS (n=14), pneumonia (n=4), or both (n=10) were at significantly increased risk to subsequently develop ACS (n=8; p=0.003), pneumonia (n=4; p=0.036) or both (n=9; p=0.011), respectively when compared to “good vaccine responders”. All HbSC patients with previous ACS (n=4) were “good vaccine responders” and none subsequently developed ACS. Of three HbSC with prior pneumonia, one “poor vaccine responder” developed pneumonia post-vaccination.
DiscussionAlthough rates of IPD in children with SCD have dropped dramatically since the introduction of pneumococcal conjugate vaccine, IPD rates in this population remain higher than in African-American children of the general population.13 Anti-pneumococcal immunity may not be optimally maintained in patients with SCD using current vaccination strategies, leaving them vulnerable to IPD. In this study, SCD patients tend to have a poor vaccine response, although HbSC mounted a better response than HbSS, particularly for serotypes 22 and 43, and a better overall vaccine response with lower incidences of acute pulmonary events. HbSC have a lower incidence of life-threatening infection because their spleen function is minimally impaired in early life. PPSV-23 elicits T cell independent IgM memory B cell in the presence of a mature spleen, but the response is attenuated in SCD related hyposplenism or asplenia.14 The loss of splenic function and ensuing reduction in opsonophagocytic activity is the most well accepted cause for the increased risk of infection with encapsulated bacteria, such as Streptococcus pneumonia. Over time, all SCD genotypes develop an increased risk for invasive bacterial infection. In view of the reported perturbations of lymphocyte phenotypes and functions in HbSS, and accounts of impaired vaccine responsiveness in individuals with HbSS, it is possible that differences in vaccine reactivity exists between patients with SCD compared to that in individuals without SCD.15
Pneumococcal capsular serotypes can vary in their immunogenicity in sickle cell patients, demonstrating susceptibility to serotype 6, 26, 234 as they are weak immunogens, while serotype 3 induces a strong immune response and does not feature as a major cause for vaccine failure. It is possible that HbSS patients’ compromised immune system may not mount an adequate response to certain serotypes compared to HbSC patients. We find differences in serotypes 22 and 43, both exclusively present in the PPSV-23 vaccine. In sickle cell populations, the incidence of pneumococcal disease caused by non-vaccine serotypes is increasing,13 although serotypes 22 and 43 did not feature as prominent causes of IPD.16,17 Serotype 43 has high carriage prevalence in children, but demonstrates low invasive potential.18,19 Serotype 22 on the other hand, was among replacement serotypes seen in children under five years of age after the introduction of the PCV-7.20–22 Selectively associated with the occurrence of meningitis, all serotype 22 isolates were susceptible to penicillin and should expectedly respond well to standard therapy.21
The overall poor vaccine response in both groups in this study, despite receiving a 2nd PPSV-23 dose is concerning, and in agreement with a previous report.5 In this study, a poor vaccine response was associated with increased acute pulmonary events, in particular for HbSS. HbSC mounted a better vaccine response than HbSS, and experienced less recurrence of complications. We postulate that poor vaccine response is a surrogate measure to poor immune status, and therefore SCD individuals are more prone to infections, an important harbinger for acute chest syndrome. It is unclear if early intervention in poor vaccine responders could avert the risk of acute pulmonary events.
A limitation of this study was that we were unable to include healthy individuals because they receive a single dose of PPSV-23 vaccination. Pre-vaccination titers were not obtained for comparison. Ideally, post-vaccination samples obtained 4–8 weeks after immunization would be used to compare to pre-vaccination samples for individual serotypes. Due to concerns for interference with the efficacy of the PCV series, and obtaining titers only after 2nd dose PPSV-23, hence it would be difficult to establish an appropriate baseline timeframe to obtain pre-vaccine titers.11,12 For epidemiologic purposes, the number of pneumococcal serotypes that are protective after a vaccine is useful to define an adequate response.11 The functionality of the anti-pneumococcal antibodies was not measured in this study as it is commercially unavailable.
In this unselected population, our data show overall poor vaccine response in SCD. Differences between HbSS and HbSC occur in immunogenic response to the pneumococcal vaccines. HbSC mount a better vaccine response than HbSS, leading to lower incidences of acute pulmonary events. Current vaccination strategies for SCD sub-types are lacking, therefore a larger study to evaluate the utility of additional vaccine boosters in children with SCD (HbSS and HbSC) compared to the general population is necessary. Poor vaccine response is concerning, thus routine monitoring of vaccine response may be beneficial.
Funding sourceThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
All authors have no financial relationships relevant to this article to disclose.
Conflict of interestAll authors have no conflict of interest to disclose.