Sensitisation to Alternaria is a cause of respiratory disease in Spain, particularly in childhood, but it is also a significant marker of the severity of this disease. Therefore, the use of an aetiological treatment (allergen specific immunotherapy) is essential, and both subjective and objective clinical parameters should be used to follow up this treatment.
ObjectiveThis open-label, uncontrolled, observational, prospective study was designed in order to study the evolution of these patients on allergen specific immunotherapy therapy in daily clinical practice and to assess the use of different monitoring tools.
Material and MethodsA total of 99 patients were included. They were monosensitised to this perennial allergen and treated with subcutaneous allergen specific immunotherapy. After one year of follow-up, these patients were assessed for the presence of symptoms, use of medication, clinical incidents, quality of life and asthma control.
ResultsAfter one year of treatment a significant fall was observed in the use of concomitant medication (β2-agonists: p=0.0278, inhaled corticosteroids: p=0.0007, anti-leukotrienes: p=0.0495), nasal symptoms (p=0.0081), quality of life (PAQLQ, p<0.0001) and asthma control (ACQ, p<0.0001). Twenty-one patients had to attend emergency department due to exacerbation of their allergic disease, and only one of them had to be admitted to hospital.
Conclusionrespiratory allergic disease due to Alternaria alternata is a disease which is hard to control, and in our daily practice, the use of specific subcutaneous immunotherapy can be of significant benefit in our paediatric patients.
Prevalence of sensitisation to Alternaria varies clearly from one country to another. Thus, it can be observed that figures vary from 3.6% in the United States1 to 20% in Spain.2,3 Sensitisation to Alternaria presents at an early age, and is most common in children.4 Some 38.3% of children with asthma in the United States presented sensitisation to Alternaria.5 Furthermore, surveys have been published with the findings that patients who are sensitised to this fungus are at a greater risk of suffering asthma,6 and that this sensitisation significantly increases the severity of this disease.7 It has also been observed that the risk of death from asthma is associated with the presence of fungal spores in the atmosphere8 and that children who are sensitised to Alternaria show respiratory symptoms and bronchial hyper-reactivity when there is an increased concentration of fungi in the atmosphere.9
In view of all the above, and from the paediatric allergy point of view, the clinical management of these patients is complex. From a therapeutic point of view, these patients require symptomatic medication as well as SIT. From a follow-up point of view, managing these patients requires the use of different tools that provide us with a detailed knowledge of the evolution of this disease.
In this study we have sought to provide a systematic approach to daily clinical practice with these patients, assessing both the efficacy of the specific immunotherapy with an extract of Alternaria 100% and the validity of the different tools used in follow-up and control. Length of follow-up was one year.
Material and methodsPatientsA total of 99 patients were included in an open-label, uncontrolled, multicentre, observational, prospective study. All patients were aged 4 to 16 years and were diagnosed with persistent moderate-severe rhinitis according to the ARIA classification10 and/or mild-moderate asthma according to the GINA classification,11 due to sensitisation to Alternaria alternata (all patients were monosensitised to Alternaria). Sensitisation was diagnosed through skin prick test (Alternaria alternata 30 HEP, ALK-Abelló, S.A.) and/or specific IgE (UniCAP®, Phadia, Sweden).
Exclusion criteria were the World Health Organisation contraindications,12 and having been treated with Alternaria immunotherapy within the previous two years.
The study was approved by the Institutional Review Boards of all the centres involved, and the written Informed Consent was obtained from all the patients’ representatives, as well as from patients over the age of 12. The patients were consecutively included over a period of three months. The study was carried out in six hospitals from northern, central, and southern Spain.
ImmunotherapyAll patients were treated with an extract of Alternaria alternata 100% (Pangramin Depot-UM®, ALK-Abelló, S.A., Madrid, Spain). This is a biologically standardised unit and the major allergen (Alt a 1) was quantified in micrograms per millilitre (maximum administered dose: 0.2 mcg Alt a 1). The immunotherapy was administered in accordance with the guidelines of the European Academy of Allergology and Clinical Immunology (EAACI)13 and the build-up regimen used was a cluster regimen (Day 1, vial 2: 0.1ml/0.2ml. Day 7, vial 2: 0.4ml/0.6ml. Day 14, vial 3: 0.1ml/0.2ml. Day 21, vial 3: 0.4ml/0.4ml). Time between doses was 30minutes and the patients remained under observation for 45minutes after the last dose in each session. Tolerance was verified in a previous study conducted by the Spanish Society of Paediatric Clinical Immunology and Allergy (SEICAP).14
ToleranceAdverse reactions to treatment with allergen specific immunotherapy (SIT) were classified according to EAACI guidelines,13 recording local and systemic reactions, as well as immediate and delayed reactions.
Clinical effectivenessThe patients were clinically assessed by scoring their symptoms and use of medication, compiled in diaries that each patient completed for 4 weeks prior to the start of treatment and for a further 4 weeks after 12 months of treatment, and by evaluating the use of concomitant medication. With regard to symptoms, we assessed nasal symptoms (itchiness, sneezing, congestion and rhinorrhea) and bronchial symptoms (cough, wheezing, difficulty breathing) using a severity score of 0-3 (0=no symptoms; 1=mild symptoms; 2=moderate symptoms; 3=severe symptoms). The use of medication was assessed by analysing the percentage of patients who required medication at baseline and after one year of treatment. The type of medication used in the study and its score is as follows: for asthma (short-acting β2-agonists: 1 point/puff; long-acting β2-agonists (LABA): 4 points/puff; inhaled corticosteroids (ICS): 2 points/puff (of 100μg of budesonide or equivalent), LABA+ICS: 8 points/puff (of 160μg of budesonide or equivalent); antileukotrienes: 8 points/pill; oral corticosteroids: 42 points/cycle). For rhinitis (oral antihistamines: 6 points/pill; nasal antihistamines: 1 point/drop/nostril; nasal corticosteroids: 2 points/neb/nostril; oral corticosteroids: 42 points/cycle).
Subjective assessment of the disease using the visual analogue scaleThe visual analogue scale ranges from 0 to 10 (0=no symptoms, 10=severe symptoms). The question used for scoring was “How is your allergy?” Furthermore, at the end of treatment, the investigator assessed how the patient's allergy was on a semi-quantitative scale (from much better to much worse) in comparison with the same period one year earlier, just before starting the treatment.
Assessment of asthma controlEvery three months, asthma control was assessed using the Asthma Control Questionnaire (ACQ)®, Juniper). This questionnaire has been used in previous studies.15
Quality of lifeBefore starting treatment and one year later, asthmatics patients completed the self-administered paediatric Asthma Quality of Life Questionnaire (Paediatric AQLQ®, Juniper).16 Four children not classified as asthmatic because they suffer from rhinitis and cough as their only asthma symptoms were however included in this analysis due to the possibility to develop asthma.
Asthma exacerbationsAt each control visit (once every three months), each investigator recorded whether patients had suffered any asthma exacerbation, defined as the need for oral corticosteroids, being seen at emergency department, at a non-scheduled allergy appointment, or missing school because of asthma.
Statistical methodsFor continuous variables; mean, standard deviation and 95% confidence intervals for the mean were calculated. In order to evaluate the variables in a non-parametrical way, median, extreme values for the distribution and interquantile rank (IQR) were provided. For categorical variables, frequencies and percentages were shown.
Changes from baselines to the visit in continuous variables were tested by the paired t-test and the non-parametric Sign Rank test. For categorical variables, changes were compared with the Mc Nemar test.
The safety of treatment was evaluated by means of the frequency of AE by their characteristics and listings.
A p value <0.05 was considered statistically significant.
ResultsSample characteristicsA total of 99 patients were included, 32 female and 67 male. The mean age of patients was 9.0±2.7 years (4-16 years) and the time of evolution of the disease was 3.9±2.2 years (1-11 years). All patients had positive skin tests to Alternaria, and specific IgE to Alternaria was measured in 65 patients, being in all of them (IgE≥class 2). The diagnosis was rhinitis in 83 (83.8%), conjunctivitis in 17 (17.2%) and asthma in 88 (88.9%. In 36 patients asthma was mild-intermittent, in 39 mild-persistent and in 13 moderate). Fifty-nine patients suffered from rhinitis and asthma and 13 from rhinoconjunctivitis and asthma.
There were a total of six drop-outs (three because the patients did not notice any improvement; one due to poor tolerance and two for unknown reasons); and one patient was withdrawn (due to a worsening of the disease).
A total of 1880 doses were administered (792 during build-up phase and 1089 during maintenance phase).
Immunotherapy toleranceA total of 81 local reactions were recorded, only 20% of which required treatment. The majority of them (73%) were delayed reactions.
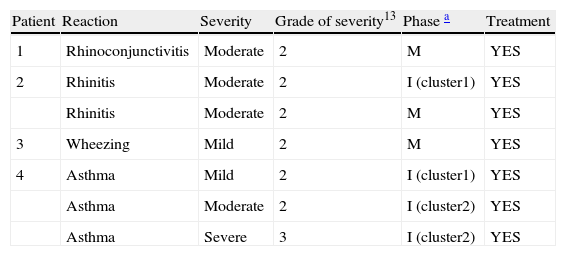
There were seven systemic reactions in four patients, one of which was severe (Table 1). All of them were delayed reactions.
Systemic reactions.
| Patient | Reaction | Severity | Grade of severity13 | Phase a | Treatment |
| 1 | Rhinoconjunctivitis | Moderate | 2 | M | YES |
| 2 | Rhinitis | Moderate | 2 | I (cluster1) | YES |
| Rhinitis | Moderate | 2 | M | YES | |
| 3 | Wheezing | Mild | 2 | M | YES |
| 4 | Asthma | Mild | 2 | I (cluster1) | YES |
| Asthma | Moderate | 2 | I (cluster2) | YES | |
| Asthma | Severe | 3 | I (cluster2) | YES |
Symptom scores fell significantly for nasal symptoms (median difference of −0.41 from baseline to final score, p=0.0087, Wilcoxon signed rank test) but for lung symptoms this reduction did not achieve statistical significance (median difference of −0.11, p=0.2395)
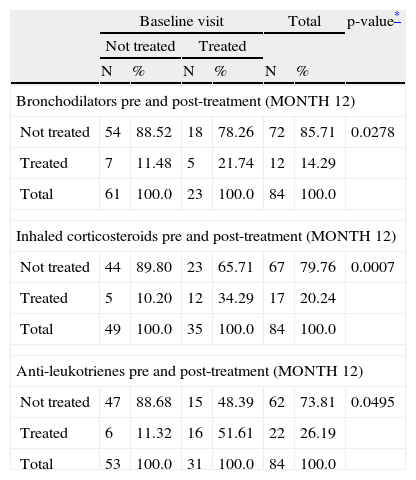
With regard to the use of medication, results showed a fall in the score for bronchodilators, inhaled corticosteroids and anti-leukotrienes (Table 2). The reduction in the use of antihistamines did not achieve statistical significance (p=0.07).
Use of medication comparing month 12 of treatment with pre-treatment baseline.
| Baseline visit | Total | p-value* | |||||
| Not treated | Treated | ||||||
| N | % | N | % | N | % | ||
| Bronchodilators pre and post-treatment (MONTH 12) | |||||||
| Not treated | 54 | 88.52 | 18 | 78.26 | 72 | 85.71 | 0.0278 |
| Treated | 7 | 11.48 | 5 | 21.74 | 12 | 14.29 | |
| Total | 61 | 100.0 | 23 | 100.0 | 84 | 100.0 | |
| Inhaled corticosteroids pre and post-treatment (MONTH 12) | |||||||
| Not treated | 44 | 89.80 | 23 | 65.71 | 67 | 79.76 | 0.0007 |
| Treated | 5 | 10.20 | 12 | 34.29 | 17 | 20.24 | |
| Total | 49 | 100.0 | 35 | 100.0 | 84 | 100.0 | |
| Anti-leukotrienes pre and post-treatment (MONTH 12) | |||||||
| Not treated | 47 | 88.68 | 15 | 48.39 | 62 | 73.81 | 0.0495 |
| Treated | 6 | 11.32 | 16 | 51.61 | 22 | 26.19 | |
| Total | 53 | 100.0 | 31 | 100.0 | 84 | 100.0 | |
The patients included in this table are only those in which basal and final data are recorded.
In the visual analogue scale, the median score fell from 5 to 1 after one year of treatment (difference of 2.5, p<0.0001, Wilcoxon signed rank test).
In the investigator's opinion, the allergic disease improved in 87% of patients, while it was the same or worse in 13%.
Assessment of asthma controlThe ACQ was completed every three months, and the following results were obtained in comparison with the baseline (median differences) (Month 3: −0.02, p=0.0031. Month 6: −0.21, p=0.0004. Month 9: −0.29, p=0.0001. Month 12: −0.43, p<0.0001).
Asthma exacerbationsA total of 21 patients had to attend the emergency department because of their allergic disease: 16 of them went once; four went twice; and one went on three occasions. Hospitalisation was only required in one case, and oral corticosteroids were required. In all cases the cause was the presence of asthma symptoms.
Because of their allergic disease, 45 patients suffered sleep disruptions, and 28 patients had to miss school (between 1 and 15 days).
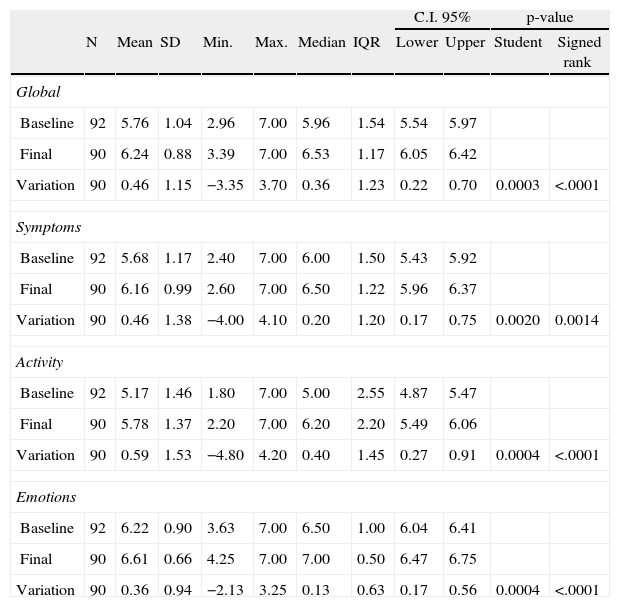
Quality of life (PAQLQ)The results are shown in Table 3. A significant improvement was observed at a global level and in the areas of symptoms, activity, and emotions.
Results of quality of life in asthmatic patients (APQLQ).
| C.I. 95% | p-value | ||||||||||
| N | Mean | SD | Min. | Max. | Median | IQR | Lower | Upper | Student | Signed rank | |
| Global | |||||||||||
| Baseline | 92 | 5.76 | 1.04 | 2.96 | 7.00 | 5.96 | 1.54 | 5.54 | 5.97 | ||
| Final | 90 | 6.24 | 0.88 | 3.39 | 7.00 | 6.53 | 1.17 | 6.05 | 6.42 | ||
| Variation | 90 | 0.46 | 1.15 | −3.35 | 3.70 | 0.36 | 1.23 | 0.22 | 0.70 | 0.0003 | <.0001 |
| Symptoms | |||||||||||
| Baseline | 92 | 5.68 | 1.17 | 2.40 | 7.00 | 6.00 | 1.50 | 5.43 | 5.92 | ||
| Final | 90 | 6.16 | 0.99 | 2.60 | 7.00 | 6.50 | 1.22 | 5.96 | 6.37 | ||
| Variation | 90 | 0.46 | 1.38 | −4.00 | 4.10 | 0.20 | 1.20 | 0.17 | 0.75 | 0.0020 | 0.0014 |
| Activity | |||||||||||
| Baseline | 92 | 5.17 | 1.46 | 1.80 | 7.00 | 5.00 | 2.55 | 4.87 | 5.47 | ||
| Final | 90 | 5.78 | 1.37 | 2.20 | 7.00 | 6.20 | 2.20 | 5.49 | 6.06 | ||
| Variation | 90 | 0.59 | 1.53 | −4.80 | 4.20 | 0.40 | 1.45 | 0.27 | 0.91 | 0.0004 | <.0001 |
| Emotions | |||||||||||
| Baseline | 92 | 6.22 | 0.90 | 3.63 | 7.00 | 6.50 | 1.00 | 6.04 | 6.41 | ||
| Final | 90 | 6.61 | 0.66 | 4.25 | 7.00 | 7.00 | 0.50 | 6.47 | 6.75 | ||
| Variation | 90 | 0.36 | 0.94 | −2.13 | 3.25 | 0.13 | 0.63 | 0.17 | 0.56 | 0.0004 | <.0001 |
Four children with rhinitis and cough as only asthma symptoms (due to this only symptom they were no classified as asthmatic) were included in this table.
As mentioned in the introduction, allergic disease due to Alternaria significantly affects children, and sensitisation to fungi (Alternaria being the commonest in our environment), is a significant risk factor in asthma severity. In our case, this situation was corroborated because 21 of our patients (23.86%) had to attend emergency department as a consequence of their allergic disease. Although only one patient required hospitalisation, this finding is quite noteworthy. It is important to stress that in all cases, the patients presented at emergency department because of exacerbation of their asthma symptoms. Some patients had concomitant respiratory infections, but in the majority of patients, the exacerbation of asthma symptoms was attributed to allergic sensitisation, because the only diagnosed cause of asthma was the Alternaria sensitisation.
Another finding that reflects the severity of the disease was that 88.8% of our patients had asthma, and the majority also had rhinitis. This finding highlights the importance of considering the management of allergic disease not only from a point of view of symptom control, but also from an aetiological point of view, because the presence of both asthma and rhinitis symptoms alike speak for the need to use a treatment that acts globally on the respiratory system, as is the case of SIT.12 However, it is noteworthy that despite all the above observations, not many studies have analysed the role of SIT in this type of sensitisation;17–22 although they have verified the tolerance and safety of Alternaria alternata extracts, in rush and conventional regimens alike,17,19 and also of clinical improvement after one year of treatment with these extracts.21,22,23
For the above reasons, the immunotherapy work group at the Spanish Society of Paediatric Clinical Immunology and Allergy decided to conduct this study, with the aim of analysing the evolution of our patients who suffer respiratory allergic disease due to sensitisation to Alternaria. The authors are fully aware of the limitations implied in the study design that they used. However, especially in the case of children, we felt that it was important to try to analyse whether our routine practice brought about a clinical improvement in our patients. This was why the decision was taken to undertake the study.
After a one year follow-up, we have been able to verify that our current therapeutic management of patients with respiratory allergic disease due to Alternaria, which includes the use of an appropriately standardised allergen extract with the majority allergen (Alt a 1) at a maintenance dose of 0.2 mcg, leads to a significant fall in the regular use of medication in our patients, although this improvement was only accompanied by an improvement in rhinitis symptoms, and not in asthma symptoms, but less medication was indeed used. It is not uncommon in clinical studies conducted with SIT to find a reduction in either symptoms or medication, but not in both parameters. However, unlike studies which only measure scores for symptoms and medication, in this study we also included the use of objective measuring tools, such as the ACQ. In our study, this last parameter revealed a significant improvement in asthma control. Since the questionnaire covers symptoms as well as the use of medication, we believe that it may be a very useful tool in our patients, especially if it is completed periodically.
We also found a significant improvement in the quality of life of our asthma patients, who accounted for the majority of the patients included in the study. This improvement, which could also be contrasted with the lack of significant improvement in the score for asthma symptoms, again speaks of the importance of using objective and scientifically-validated tools in routine clinical practice.
Finally, we believe that it is important to stress that both the visual analogue score completed by the patients, as well as the investigator's assessment, coincided in finding a notable improvement in the patients’ situation, one year after starting treatment.
To conclude, respiratory allergic disease due to Alternaria is a disease that is hard to control, and in our daily practice, the use of specific subcutaneous immunotherapy can be of significant benefit in our paediatric patients. To follow up the clinical evolution of these patients, it is essential to routinely use objective tools that will provide us with knowledge of how the disease is being controlled and of the evolution of our patients’ quality of life.
Conflict of interestThe authors have no conflict of interest to declare.