INTRODUCTION
Specific immunotherapy (SIT) is currently acknowledged as a key therapeutic approach for patients with respiratory allergies. SIT is a "biological response modifier", acting on an upstream phase of the immune response. It therefore not only relieves symptoms in the long run, and the need for anti-allergic drugs, but modifies the natural course of the disease, hampering or even blocking its progression 1,2. The causes of that are yet not clear because the immunological changes induced by SIT are complex as it interferes with pathophysiologic mechanism responsible for the release of mediators and subsequent inflammatory changes 3-10. The mast cells and eosinophils play a key role in this process and a possible activity of the SIT on these cells has been assessed in a previous study 11.
The clinical efficacy of SIT has been stated some years ago in two reviews of the literature by a panel of WHO and EAACI experts 1,2, as reported in their Position Papers, which also sets out guidelines for prescribing and carrying out the treatment. An interesting novelty in these papers is the validation and acceptance of non-injective administration routes for SIT (sublingual and intranasal), which are recognised as useful and can be prescribed in allergological practice. The main aim of sublingual immunotherapy (SLIT) is safety in use, and they have been under investigation for at least 20 years, so there is considerable data on their efficacy and safety. Aim of the present study, that was carried out in Portugal from 2001 to 2003, was to confirm, in a population of allergic patients with seasonal (intermittent) rhinoconjunctivitis with or without mild intermittent or mild persistent asthma, the clinical efficacy and the safety of a sublingual preseasonal oromucosal IT performed with a monomeric allergoid (allergoid SLIT) for grass pollens. Secondary endpoint was to evaluate the effect of this kind of immunotherapy on nasal reactivity after allergen nasal challenge.
MATERIAL AND METHODS
Study design
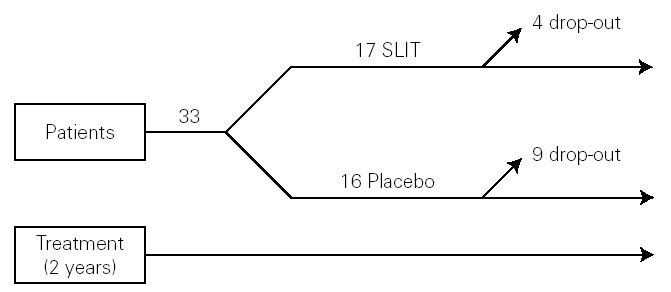
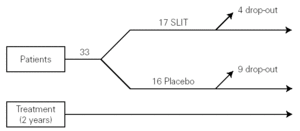
The study was monocentric, randomised, double-blind, placebo-controlled. Patients were selected and randomly allocated into two groups: one took for two years the active treatment (allergoid SLIT) and the other placebo. Both groups received all the necessary symptomatic pharmacological treatment throughout the two-year period of the trial (fig. 1). A run-in phase was not planned.
Figure 1.--Study design.
Patients
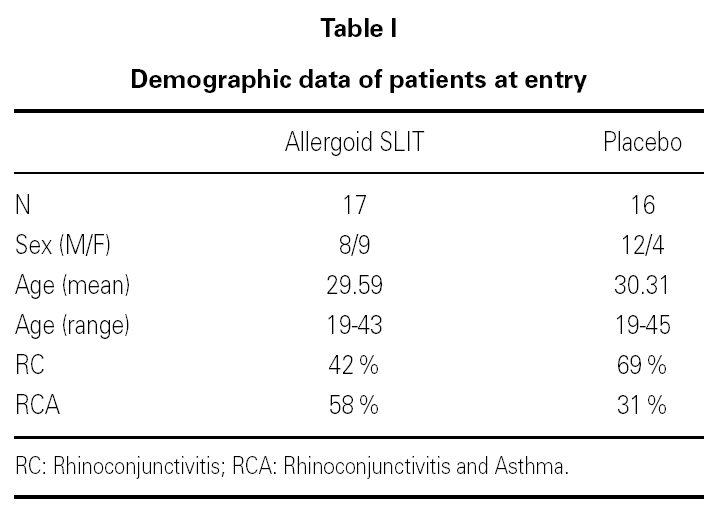
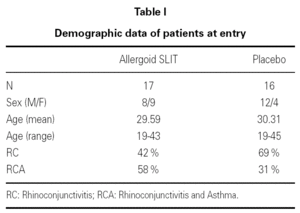
Thirty-three outpatients (20 men and 13 women, mean age: 30 years; range: 19-43) with a clinical history of seasonal (intermittent) rhinoconjunctivitis with (58 %) or without (42 %) mild intermittent or mild persistent asthma since at least two years and attending our allergy centre were enrolled into the study after obtaining their informed consent and the approval of the ethical committee of the University Hospital (table I). Enrolment of the patients was preseasonal followed by a seasonal visit in May and an end-of-the-year visit in October. Patients were followed during two consecutive years. All patients were sensitized to grass pollens, as confirmed by skin prick test (Lofarma S.p.A., Milan, Italy) and RAST (CAP System EIA, Pharmacia) performed with a panel of common allergens. Subjects suffering from severe systemic and/or autoimmune diseases or acquired/congenital immune deficiencies, past or current malignancy, neurological or psychiatric disorder requiring major psychodrugs, receiving chronic systemic corticosteroid or beta-blocking treatments were not enrolled, nor were pregnant women. Patients were consecutively allocated in randomised manner, into two groups receiving IT plus rescue pharmacotherapy (n = 17) or placebo plus rescue pharmacotherapy (n = 16).
Investigational IT and rescue pharmacotherapy
The IT was performed with a mixture of chemically modified allergenic extracts (carbamylated monomeric allergoid) from grass pollens (Holcus lanatus 33 %, Phleum pratense 33 %, Poa pratensis 33 %) incorporated in oromucosal tablets (Lais®, Lofarma S.p.A., Milan, Italy). The tablets had to be dissolved in the mouth in 1-2 minutes before swallowing. The allergoid is used in order to reduce the allergen's ability to react with IgE, while keeping its antigenicity intact. It can therefore still cause useful immunological changes such as the Th2/Th1 switch, reducing lymphocyte proliferation of the allergen. This aim was reached by developing a chemical method, the carbamilation, which permits targeted changes to amino groups (mainly lysine) without affecting the molecular size, unlike what happens to conventional polymer allergoids obtained by conventional aldehyde treatment 12. Since it is small, this allergoid offers good transmucosal absorption rate, which is exploited in this type of therapy. Moreover, differently from allergen, it resists to enzyme attack and remain intact even at gastrointestinal level. The product was titrated in Biological Units (Allergen Units, AU) 13 and standardised in potency by RAST-inhibition procedure in comparison to an in house preparation reference (IHR). The rationale for the formulation in tablets is dual: excellent chemical stability, and consequent good safety. Acute and subchronic toxicity studies in animal models, using carbamylated monomeric allergoids, have shown that it is well tolerated. Administration of the product by the oromucosal route, instead of to the restricted sublingual area, was defined on the basis of pharmacokinetic investigations in man, which showed that the product kept under the tongue is not absorbed systemically and does not reach the circulation until the tablet was swallowed. However, the whole of the buccal mucosa takes it up, consequently also involving the mucosal-associated lymphoid tissue (MALT) 14-16. The treatment is available at the following dosages: 25, 100, 300, and 1,000 AU. The initial therapy consisted in a traditional scheme lasting 14 weeks. The maintenance therapy was performed at the posological scheme of two tablets of 1000 UA per week, given pre-seasonally till May. The prescribed drugs (rescue medication) were: oral and local antihistamines (loratadine or cetirizine 10 mg/day), topical antihistamines, β2-agonist spray, inhaled corticosteroids, anti-leukotrienes and oral corticosteroids.
Symptom/medication score.
Patients were instructed to record symptoms and any medications taken on a daily diary card during the pollen season, from April to July. At each scheduled visit they were collected and checked. The monthly symptom score was obtained by rating symptoms reported daily by patients according to the following scale: 0 no symptom; 1 mild; 2 moderate; 3 severe symptoms. The medication recorded by patients in their diary cards was rated on the following scale: local or systemic antihistamines: one point per day; local corticosteroids: two points per day; inhaled b2-agonists: one point for each daily administration; anti-leukotrienes: one point per day; oral corticosteroid: three points per day.
Nasal challenge test
Nasal reactivity of each patient was analysed by specific (grass mix) challenge test. After an adaptation period of at least 30 minutes the baseline value was determined 15 minutes after the application of the control capsules (lactose) by a specific device. The reactivity to the mixture of grass allergen was measured after application of a fixed dose of 120 AU. The test was assessed as positive if the decrease of nasal flow was greater than 40 % assessed by rhinomanometry (Rhinospir-164®" Markos Mefar).
Statistical analysis
Since the clinical scores might be non-normally distributed, the Mann Whitney and the Chi-square tests for intergroup comparison and the Wilcoxon test for intragroup comparison were used. P values less than 0.05 were considered significant.
RESULTS
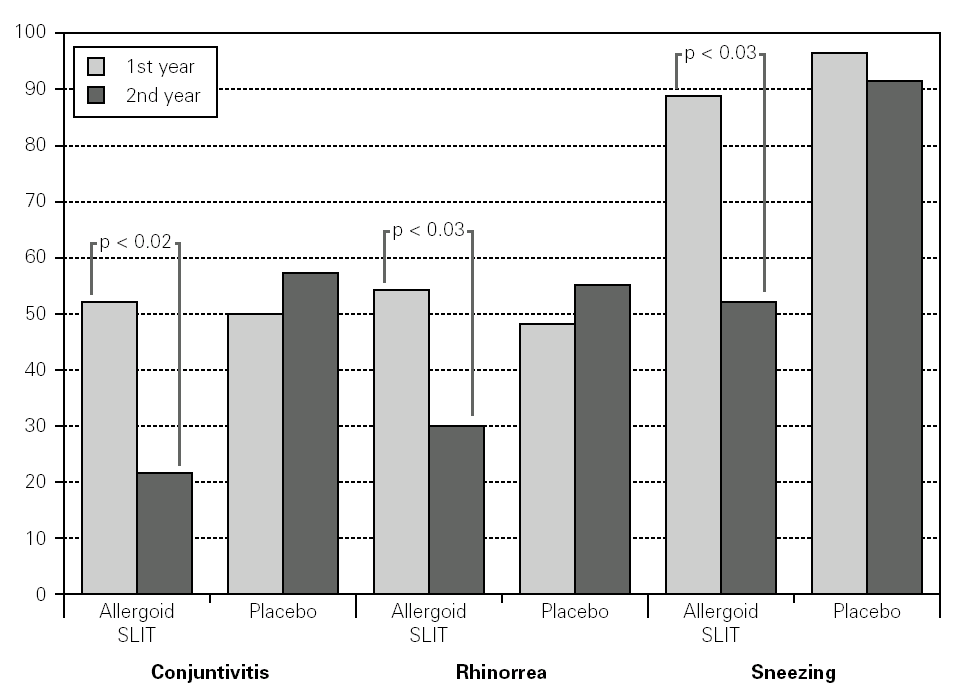
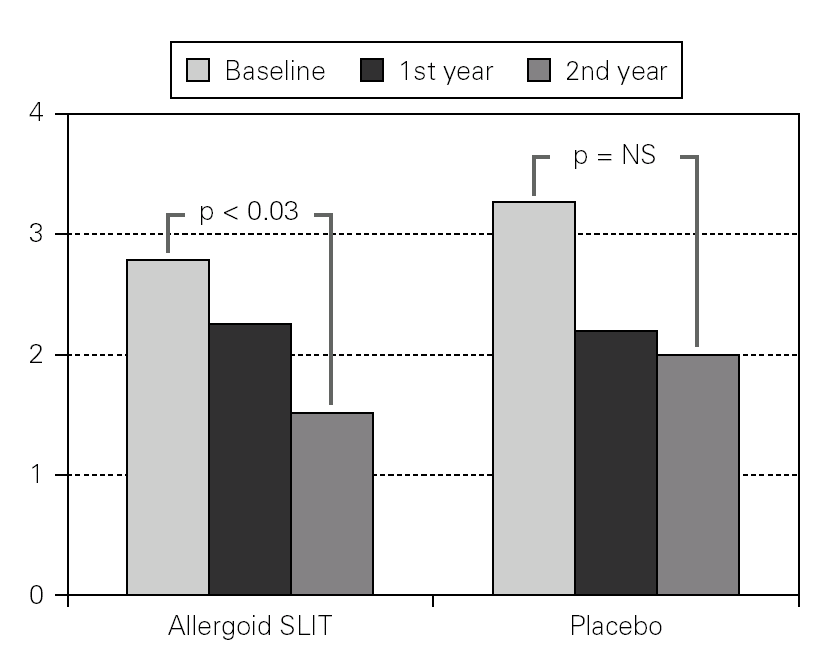
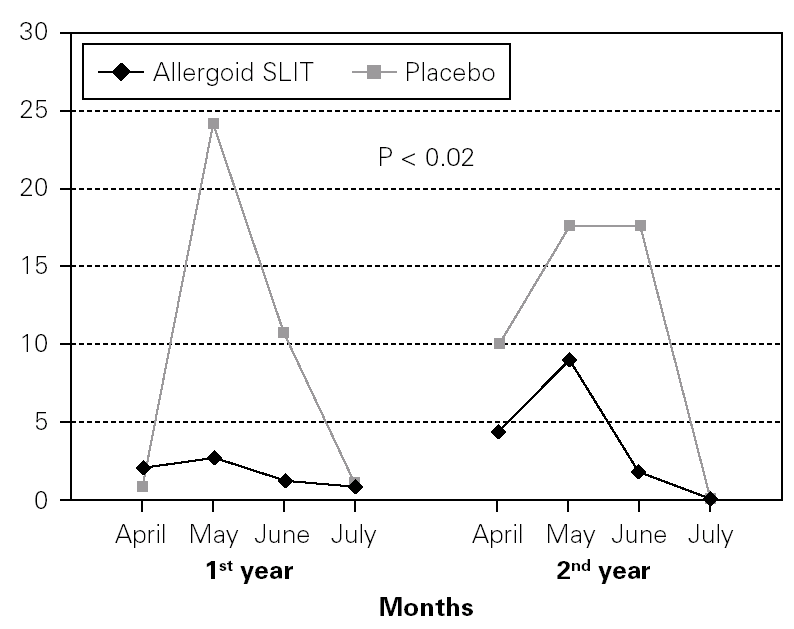
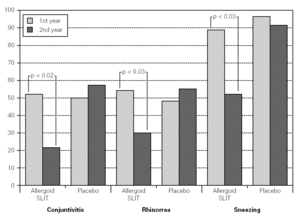
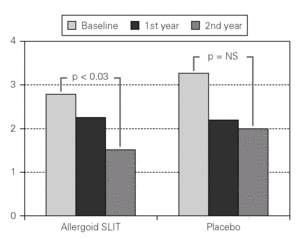
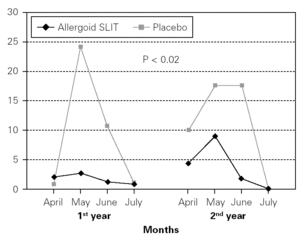
Twenty patients out of the 33 enrolled (60.6 %) completed the study. The patients treated with the allergoid SLIT showed a statistically significant improvement at the second year of treatment as far as the symptoms "conjunctivitis" and "rhinorrea" and "sneezing" were concerned while this improvement was not seen or it was not statistically significant in the placebo group (fig. 2). The nasal provocation test (TPN) showed an improvement of the nasal reactivity in both the groups, but it was statistically significant at the second year, in comparison to the baseline values, only in the active group (fig. 3). The consumption of inhaled steroids was statistically higher in the placebo than in the allergoid SLIT group (fig. 4). As far as the tolerability is concerned, the patients' judgement about that was very good both for the created patients and the placebo group. Only two mild local adverse events were observed with the allergoid SLIT (none with placebo). They did not require the interruption of the therapy.
Figure 2.--Mean scores for the symptom "conjunctivitis", "rhinorrea" and "sneezing" and in active and placebo group during the first and second pollen season. Columns represent the sum of the mean monthly values.
Figure 3.--Mean scores for the nasal reactivity at the nasal provocation test in active and placebo group at baseline, after one and two years of treatment.
Figure 4.--Steroid consumption in active and placebo group during the first and the second pollen season. Mean monthly scores
DISCUSSION
Our results show that a two-year regimen of IT with the carbamylated allergoid SLIT is effective in reducing the rhinitic symptoms and the drug consumption during the grass pollen season in a population of adults patients. This clinical improvement was seen only in the active group and only after the second year of treatment. This aspect confirm the WHO and EAACI documents, i.e. state that it is necessary to perform at least a two years course of IT to get a relevant clinical benefit 1,2 even if the reasons of that are not still completely elucidated 18. Furthermore, most of the published double-blind, placebo-controlled studies about SLIT do not last more than one year, with only six clinical trials, out of the 29 published so far, lasting two years 19. Yet, the fact that this improvement was seen only with the allergoid SLIT and not with the placebo clearly proves that the effect is caused by the vaccine. Besides there was a clinically significant difference at the second year between the active and the placebo group even if this did not reach the statistical significance due to the small number of patients that completed the two-year treatment. The previous controlled clinical studies performed both in adults and children allergic to grass pollen with the allergoid SLIT in tablets showed a satisfactory efficacy level associated with a good tolerability profile and an optimal compliance 20-22. Our findings go in the same direction of these data, even if further confirmation by investigating a greater number of patients would be useful.
CONCLUSIONS
The results of this double-blind, placebo-controlled study allow us to conclude that a 2-year treatment with the allergoid SLIT is effective and safe. When administered in association with pharmacotherapy it improves the rhinitic symptoms and decreases the need of employing inhaled corticosteroids.
Correspondence:
M.E. Bruno, MD
Medical Department. Scientific Direction.
Lofarma S.p.A.
Viale Cassala 40. 20143 Milano. Italy.
E-mail: lofscie@lofarma.it