The clinical relevance of elevated basal serum tryptase (eBST≥11.4ng/ml) often remains unclear.
MethodsBST was assessed in 15,298 patients attending our outpatient clinic. Frequency and severity of anaphylaxis was compared in 900 patients with eBST and 900 patients with normal BST. The prevalence of eBST was evaluated in patients with drug reactions, urticaria, gastrointestinal symptoms or venom allergy. Mast cell-associated symptoms were recorded prospectively in 100 patients with eBST and 100 controls using a standardised questionnaire.
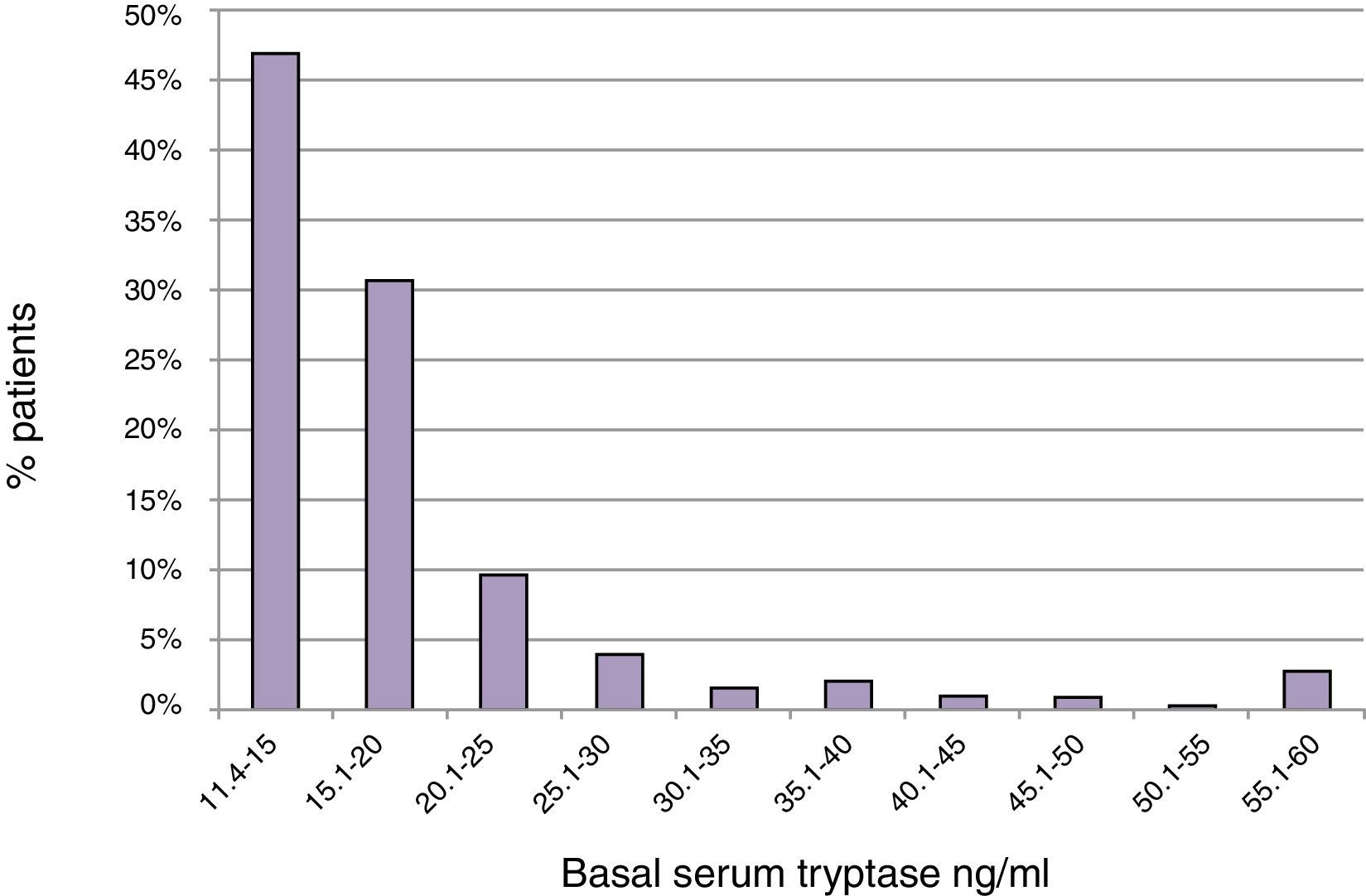
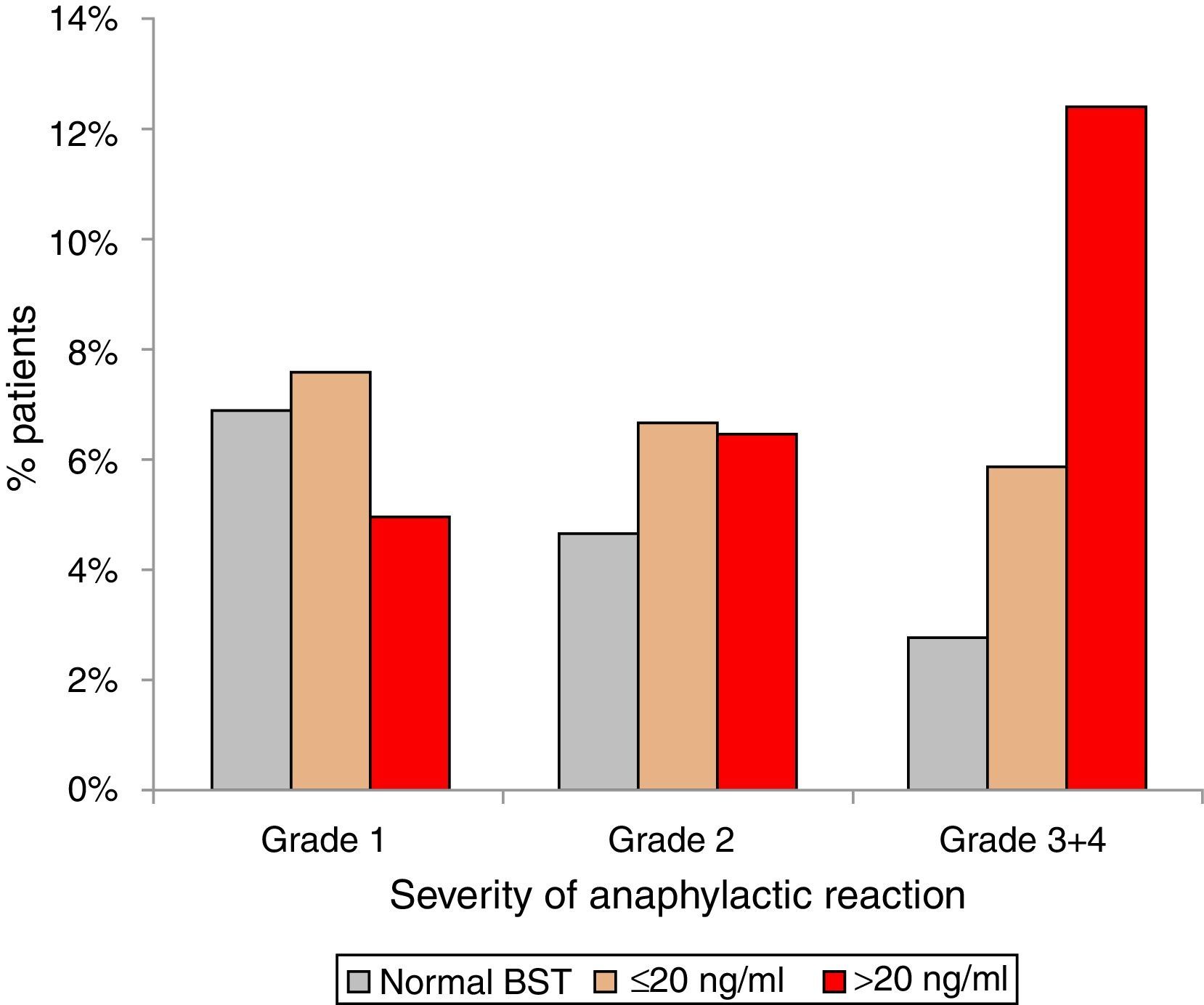
Results5.9% (n=900) of 15,298 patients had eBST ≥11.4ng/ml (mean 20±21ng/ml, 11.4–390ng/ml). In 47% of them BST was <15.0 and in 78% <20.0ng/ml. In patients with normal BST (4.5±2.1ng/ml), mean levels increased continuously with age (0.28ng/ml per decade; p<0.001). Fatigue, meteorism, muscle/bone ache, vertigo, tachycardia, flush, palpitations, diarrhoea and oedema were associated with eBST (p<0.05 to <0.0001) without significant differences between slightly (11.4–20ng/ml) or strongly (>20ng/ml) eBST. eBST was significantly associated with adverse reactions to drugs (34%), radio contrast media (15%) and insect stings (24%) (p<0.05). Anaphylaxis was more common in patients with eBST (21% vs. 14%, p<0.001). The relative role of insect stings, drugs and food as the most important triggers was similar in patients with elevated and normal BST. Severe reactions (grade 3/4) occurred most often in subjects with BST >20ng/ml (BST <11.4mg/ml: 2.8%; 11.5–20ng/ml: 5.9%; >20ng/ml: 12.4%).
ConclusionsIn clinical practice it appears reasonable to assess BST, besides after anaphylactic reactions also in patients suffering repeatedly from vertigo, flush, tachycardia, palpitations, oedema and nausea. Even patients with slightly eBST have a higher risk of anaphylaxis and experience more severe reactions.
Assessment of basal serum tryptase (BST) is part of the routine diagnostic work-up after anaphylactic reactions to insect stings.1–3 BST values exceeding 20ng/ml are not only associated with an elevated risk for anaphylaxis4,5 but represent a minor criterion for mastocytosis.6 However, the clinical relevance and the risk profile of slightly elevated BST values ranging between the cut off level 11.4ng/ml and 20ng/ml are still unclear. In patients with Hymenoptera venom allergy, an elevated risk for severe anaphylactic reactions has been proposed starting with a BST as low as 5ng/ml, a value far below the cut off of 11.4ng/ml.7
There is a lack of cardinal symptoms helping to diagnose mastocytosis or mast cell activation syndromes. This may be mostly explained by the heterogeneous and unspecific symptomatology of these diseases. Only cutaneous mastocytosis with its characteristic Darier's sign can be easily diagnosed clinically. On the other hand, episodic cutaneous symptoms such as flushing, pruritus or urticaria, abdominal complaints with nausea, diarrhoea and cramps as well as cardiovascular symptoms such as hypotension or tachycardia are characteristically found in patients with mast cell diseases.4,8,9 These symptoms, especially when occurring repeatedly and/or simultaneously, should give reason to assess BST.
The most common commercially available testing kit for tryptase (Thermo Fisher Scientific, Uppsala, Sweden) measures alpha as well as beta tryptase (“total” tryptase).10 Immature precursors of alpha and beta tryptase are continuously released into the circulation giving a gross estimate on the bodies mast cell load. However, only mature beta tryptase is released in the course of IgE- and non-IgE-mediated mast cell degranulation. Currently, a selective commercial assay for mature beta tryptase is not available, therefore the recent literature has been focusing on “total serum tryptase”.
The aims of this study were to explore the frequency of elevated BST among routine “allergy” patients of a large outpatient allergy clinic and their distribution with respect to age and gender. In addition, symptoms in patients with elevated BST were assessed in a prospective as well as retrospective manner in order to identify cardinal symptoms helpful in detecting patients with mast cell diseases. Finally, the clinical relevance of slightly (11.4–20ng/ml) and strongly (>20ng/ml) elevated BST levels with respect to the risk profile of patients (including anaphylaxis) was evaluated.
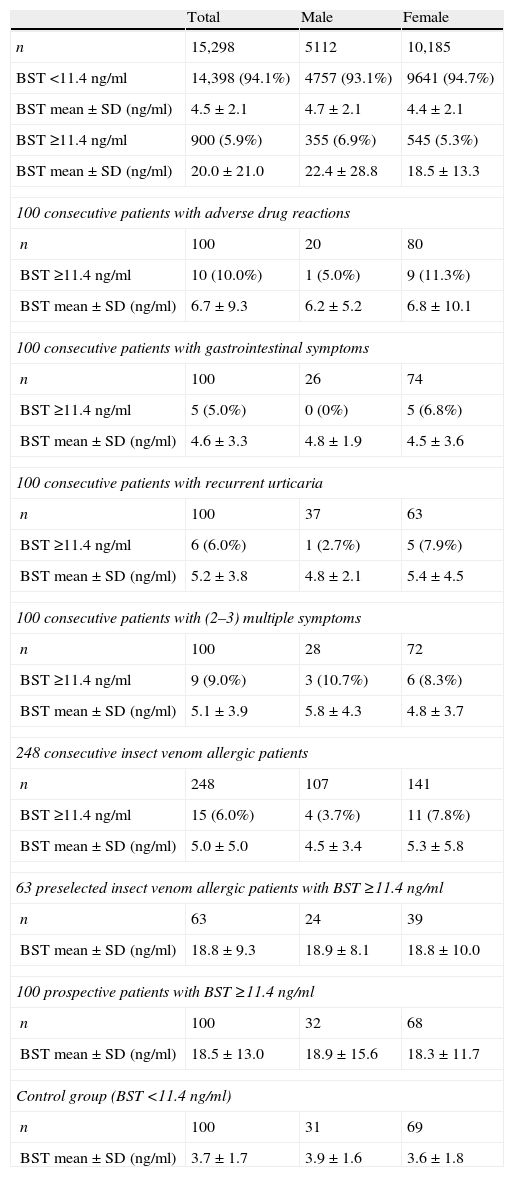
Materials and methodsThis manuscript consists of a retrospective and a prospective part. Table 1 gives an overview of all patient groups investigated.
An overview of all patient groups investigated.
| Total | Male | Female | |
| n | 15,298 | 5112 | 10,185 |
| BST <11.4ng/ml | 14,398 (94.1%) | 4757 (93.1%) | 9641 (94.7%) |
| BST mean±SD (ng/ml) | 4.5±2.1 | 4.7±2.1 | 4.4±2.1 |
| BST ≥11.4ng/ml | 900 (5.9%) | 355 (6.9%) | 545 (5.3%) |
| BST mean±SD (ng/ml) | 20.0±21.0 | 22.4±28.8 | 18.5±13.3 |
| 100 consecutive patients with adverse drug reactions | |||
| n | 100 | 20 | 80 |
| BST ≥11.4ng/ml | 10 (10.0%) | 1 (5.0%) | 9 (11.3%) |
| BST mean±SD (ng/ml) | 6.7±9.3 | 6.2±5.2 | 6.8±10.1 |
| 100 consecutive patients with gastrointestinal symptoms | |||
| n | 100 | 26 | 74 |
| BST ≥11.4ng/ml | 5 (5.0%) | 0 (0%) | 5 (6.8%) |
| BST mean±SD (ng/ml) | 4.6±3.3 | 4.8±1.9 | 4.5±3.6 |
| 100 consecutive patients with recurrent urticaria | |||
| n | 100 | 37 | 63 |
| BST ≥11.4ng/ml | 6 (6.0%) | 1 (2.7%) | 5 (7.9%) |
| BST mean±SD (ng/ml) | 5.2±3.8 | 4.8±2.1 | 5.4±4.5 |
| 100 consecutive patients with (2–3) multiple symptoms | |||
| n | 100 | 28 | 72 |
| BST ≥11.4ng/ml | 9 (9.0%) | 3 (10.7%) | 6 (8.3%) |
| BST mean±SD (ng/ml) | 5.1±3.9 | 5.8±4.3 | 4.8±3.7 |
| 248 consecutive insect venom allergic patients | |||
| n | 248 | 107 | 141 |
| BST ≥11.4ng/ml | 15 (6.0%) | 4 (3.7%) | 11 (7.8%) |
| BST mean±SD (ng/ml) | 5.0±5.0 | 4.5±3.4 | 5.3±5.8 |
| 63 preselected insect venom allergic patients with BST ≥11.4ng/ml | |||
| n | 63 | 24 | 39 |
| BST mean±SD (ng/ml) | 18.8±9.3 | 18.9±8.1 | 18.8±10.0 |
| 100 prospective patients with BST ≥11.4ng/ml | |||
| n | 100 | 32 | 68 |
| BST mean±SD (ng/ml) | 18.5±13.0 | 18.9±15.6 | 18.3±11.7 |
| Control group (BST <11.4ng/ml) | |||
| n | 100 | 31 | 69 |
| BST mean±SD (ng/ml) | 3.7±1.7 | 3.9±1.6 | 3.6±1.8 |
BST: basal serum tryptase.
From 2004 to 2012 BST was assessed in a total of 15,298 patients (5112 male, 10,186 female, mean age 52.2±18.5years, range 1–99years). In case of multiple assessments in one patient, the very first value was used. Data were analysed with regard to gender, age and frequency of elevated BST values. The cut-off level for elevated tryptase was 11.4ng/ml following the recommendations of the manufacturer.
Anaphylaxis and risk assessmentThe frequency and severity of anaphylactic reactions was analysed in 900 patients with elevated BST and 900 randomly chosen patients with normal BST. Reactions were counted as anaphylactic only when having appeared immediately (i.e. within 15min) after a possible causative event and when other causes have been ruled out. Anaphylactic reactions were graded according to Ring and Messmer.11
Prevalence of elevated BST in patients with specific symptomsIn order to assess the prevalence of elevated BST in certain clinical conditions, 100 consecutive patients each with specific symptoms such as (1) drug intolerance; (2) recurrent gastrointestinal symptoms (diarrhoea, meteorism, abdominal cramps); (3) multilocular symptoms (2–3 different symptoms such as diarrhoea, abdominal cramps, debilitating fatigue, headache, vertigo, muscle and bone ache); or (4) chronic-recurrent urticaria, were analysed.
In a retrospective analysis, 248 consecutive patients with Hymenoptera venom allergy experiencing local swellings or systemic reactions after an insect sting were analysed to evaluate the frequency of elevated BST levels and their distribution in regard to the severity of the sting reaction. Furthermore, 63 venom-allergic patients with elevated BST were selected to study the relationship between severity of the sting reaction and the magnitude of the elevated BST level.
Clinical characterisation of patients with elevated BSTFrom January until October 2012, potentially mast cell-associated symptoms were assessed in 100 patients with elevated BST (≥11.4ng/ml) using a detailed, standardised questionnaire. All patients signed a written informed consent. BST had been assessed initially because of cutaneous symptoms (urticaria, flush, pruritus, oedema; n=28), recurrent diarrhoea and other gastrointestinal symptoms (n=26), adverse drug reactions (n=23) or reactions after insect stings (n=11). In a few cases, anaphylactic reactions, hypotension or headache had led to BST assessment. Four patients already suffered from a known cutaneous or indolent systemic mastocytosis. A control group consisted of 100 age- and gender-matched persons with allergic rhinoconjunctivitis. In order to avoid any systematic error when comparing the prevalence of particular symptoms in patients and controls, the initial symptoms having led to the first BST assessment were not considered.
Items of the questionnaire covered symptoms regarding the skin, gastrointestinal tract, respiratory tract and cardiovascular system as well as psychological and the general condition. In order to be eligible for inclusion to the study, the symptoms had to appear episodically and recurrently. Reactions to insect stings and drug intake were also documented. The aim of this investigation was to identify cardinal symptoms characterising a mast cell disease and resulting in a more targeted and economic in vitro diagnostic approach. Another issue was to clarify whether a slightly elevated BST (<20ng/ml) represents an independent risk factor for anaphylactic reactions.
BST assessmentBST was measured using Phadia ImmunoCAP® (Thermo Fisher Scientific, Uppsala, Sweden). This assay measures total serum tryptase consisting of the inactive precursors of α- and β-tryptase and the mature α- and β-tryptase. According to the manufacturer, the cut-off for elevated serum tryptase levels was set to 11.4ng/ml. Intra- and inter-assay variation in our lab were <1% and <5%, respectively.
Statistical analysisMultiple and univariate regression analysis were performed to demonstrate the partial impact of age and gender on BST. 95% confidence intervals (95% CI) represent the distribution of the regression coefficient. SPSS Statistics for Windows, Version 17.0 (SPSS Inc., Chicago) was used for the multiple regression analysis. For statistical comparison of symptom frequencies, prevalence of anaphylaxis and anaphylaxis triggers, the chi2-test was applied. Two-sided t-test or the Mann–Whitney u-Test was used to compare metric data of different groups. The significance level was set to p<0.05.
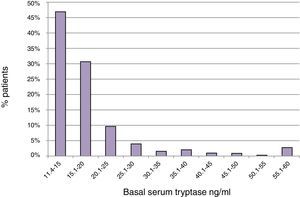
ResultsBST determination in routine patientsBST was assessed in 15,298 patients of whom 900 (5.9%) had elevated BST values (20±21ng/ml; range: 11.4–390ng/ml). The mean age of these patients was 52.2±18.5 years with a range of 3–99 years. The mean BST value was 22.4±28.8ng/ml in men (n=355), and 18.5±13.3ng/ml in women (n=545) (p<0.05) (Table 1). Mean tryptase levels were significantly lower in children 1–10 years of age (15.4±5.5, n=21) and adolescents 11–18 years of age (16.3±7.6, n=30) as compared to adults (20.3±21.5, n=849, p<0.01). The proportion of tryptase levels >20.0ng/ml was lowest in children (4.8%), intermediate in adolescents (13.3%), and highest in adults (23.0%). Overall, 46.9% of the elevated BST values ranged between 11.4 and 15.0ng/ml and only 22.4% were >20.0ng/ml (Fig. 1).
The most frequent causes for an initial determination of BST in children, adolescents and adults were cutaneous symptoms including urticaria, angio-oedema, flush, and pruritus (41/29/32%), adverse reactions after insect stings (7/18/16%) or drug intake (11/14/15%), relapsing gastrointestinal complaints like diarrhoea and meteorism (0/14/17%), cardiovascular symptoms (palpitations, hypotension, collapse) (0/0/6%), and headaches (4/4/5%). In 3.7% of patients a diagnosis of mastocytosis has already been established beforehand. Allergic rhinitis or asthma alone had been no primary reason for BST assessment.
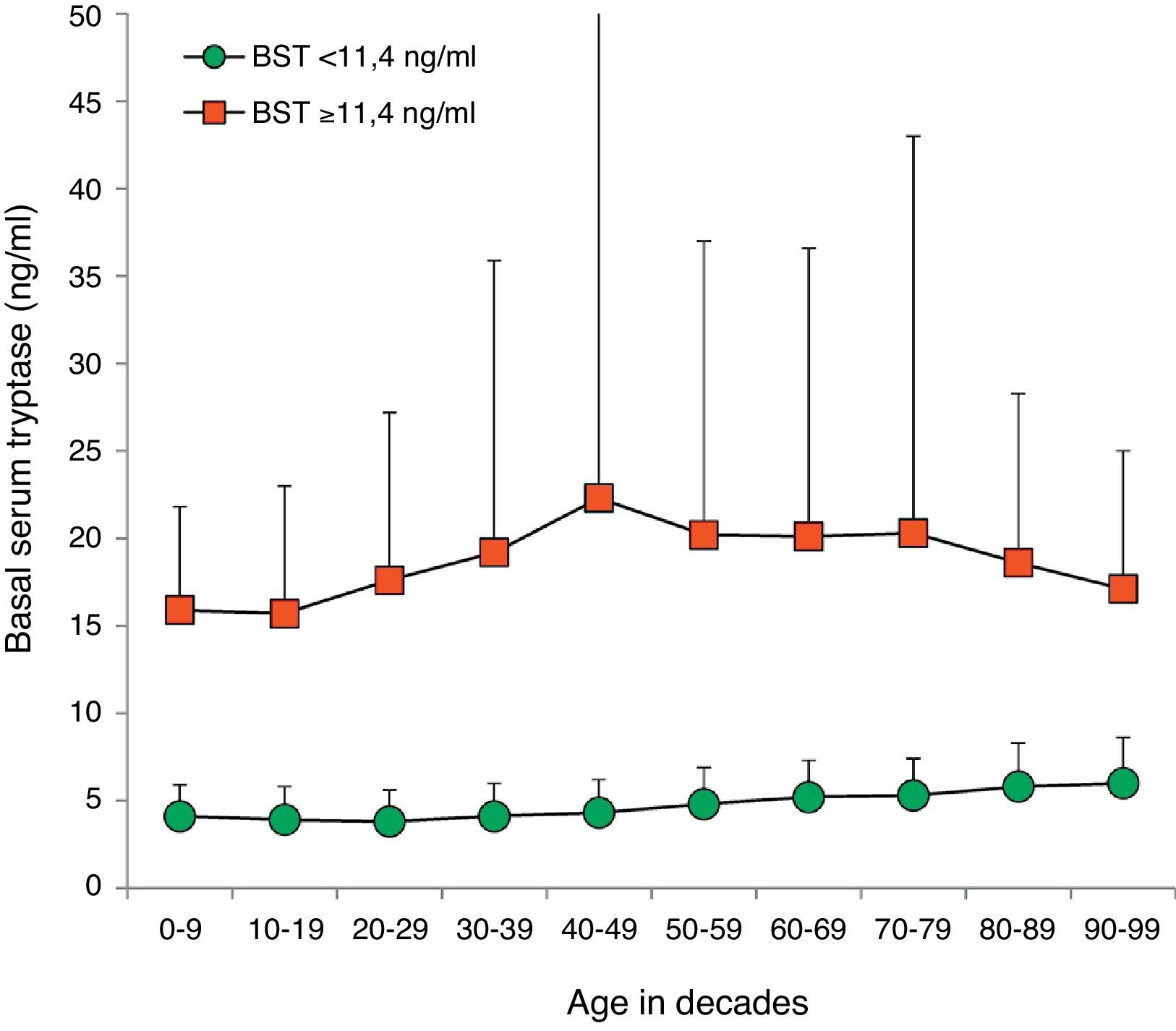
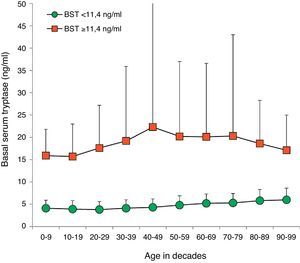
BST values were within the normal range in 14,398 (94.1%) patients (4.5±2.1ng/ml, 9641 female, 4757 male, mean age 45.0±18.5years, range 1–98years). Multiple as well as univariate regression analysis showed an influence of age and sex on BST with an age-dependent increase of 0.28ng/ml per decade (95% CI 0.26–0.30; p<0.001) (Fig. 2). Men showed higher mean BST levels, exceeding women by 0.38ng/ml (multivariate, 95% CI 0.31–0.45; p<0.001) or 0.30ng/ml (univariate, 95% CI 0.23–0.37, p<0.001) depending on the regression analysis model.
Distribution of BST depending on age. Green circles: 14,398 patients with BST <11.4ng/ml; red squares: 900 patients with BST ≥11.4ng/ml; mean±standard deviation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
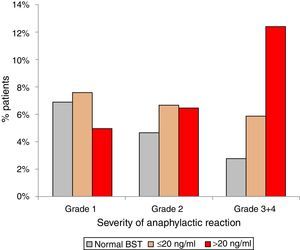
Anaphylactic reactions occurred in 14% (129/900) of randomly selected patients with normal BST and in 21% (189/900) patients with elevated BST (p<0.001). In addition, patients with elevated BST suffered from more severe reactions. Anaphylactic reactions among patients with normal BST were severe (grade 3+4) in 2.8% (25/900), but in 7.3% (66/900) of patients with elevated BST (p<0.0001). Stratifying for slightly (<20ng/ml) and strongly (>20ng/ml) elevated BST revealed severe anaphylaxis in 5.9% (41/699) of those with slightly elevated BST and 12.4% (25/201) of those with strongly elevated BST (p<0.001) (Fig. 3).
Severity of anaphylaxis according to Ring & Messmer11 in patients with normal BST <11.4ng/ml (n=129), BST 11.4–20ng/ml (n=141), and BST >20ng/ml (n=48).
There was no difference concerning the elicitors of anaphylaxis in respect of normal and elevated BST levels. The three main elicitors of anaphylaxis in adults were insect stings (62% vs. 63%), followed by drugs (22% vs. 27%), and foods (10% vs. 9%). In children (n=14), the most important triggers were foods (50% vs. 25%) and insect stings (33% vs. 62%), whereas drugs were less important (17% vs. 13%).
BST in selected patient groupsThe prevalence of elevated BST was assessed in 100 consecutive patients each with either drug reactions, gastrointestinal symptoms, multiple symptoms, or urticaria. Elevated BST was found most frequently in the group with adverse drug reactions (10%), followed by patients with multilocular symptoms (9%), chronic-recurrent urticaria (6%) and gastrointestinal symptoms (5%).
Out of 248 consecutive patients with insect venom allergy, 13 (5.2%) had an elevated BST. Patients with grade 3 and 4 reactions had significantly more often elevated BST levels (6/45, 13.3%, p<0.05) and significantly higher mean BST values (7.5±9.9ng/ml, p<0.05) compared to patients with large local reactions (2/72, 2.8%, mean 4.5±3.1ng/ml) or mild systemic reactions (5/131, 3.8%, mean 4.4±2.6ng/ml).
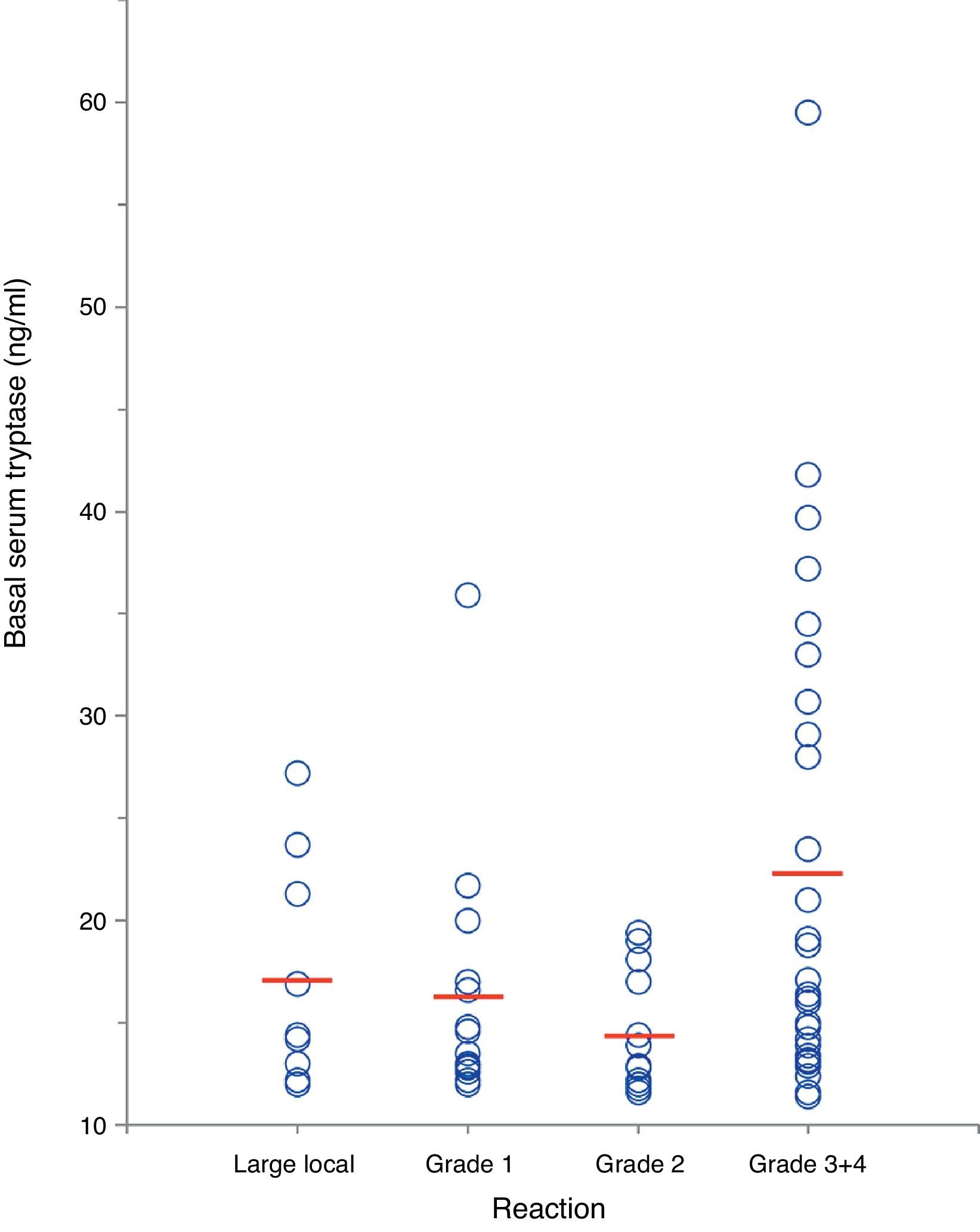
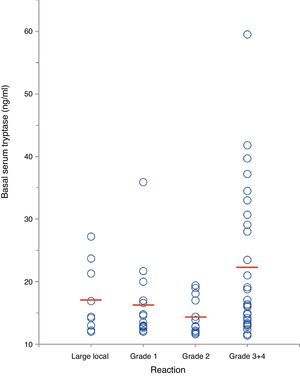
In a separate analysis of 63 insect venom-allergic patients with elevated BST, significantly higher BST levels were found in patients with severe anaphylactic reactions (22.4±11.8ng/ml, n=28) compared to those with local reactions (17.2±5.5ng/ml, n=9) or mild systemic reactions (15.6±5.1ng/ml, n=26; p<0.01) (Fig. 4). In addition, patients with severe reactions had a tendency of being older. Alvarez-Twose et al.12 recently proposed a simple clinical scoring system to estimate the likelihood of clonal vs. non-clonal mast cell disease among subjects with anaphylaxis and elevated BST. Applying this score to the 54 patients with systemic sting reactions, 23/24 (96%) of those with syncope after the sting had a score ≥2 (indicative of clonality) in contrast to only 4/30 of patients (13%) with mild reactions.
Correlation between severity of insect sting reaction and BST in 63 hymenoptera venom-allergic patients with elevated BST. The red lines represent mean BST levels. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
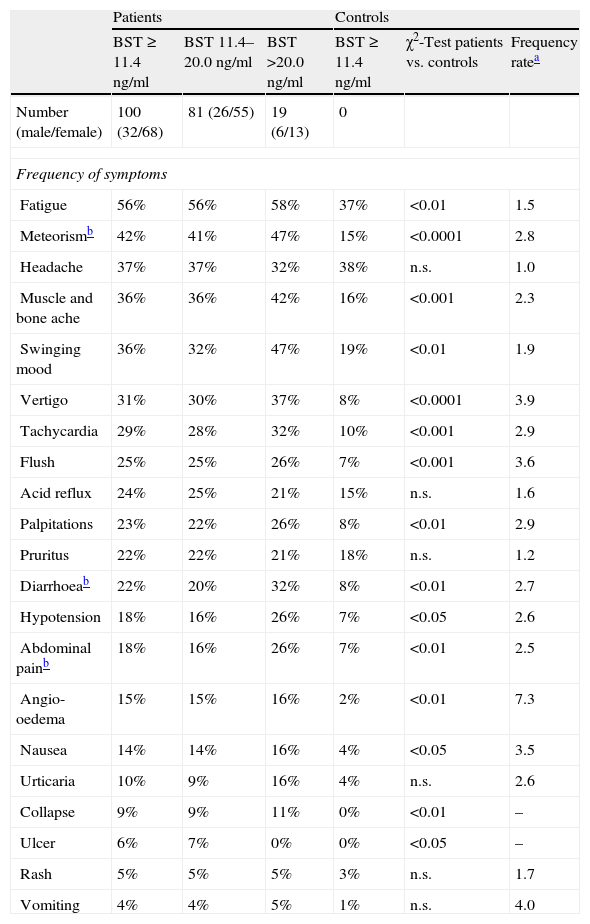
The frequency and range of potentially mast cell-associated symptoms was studied in 100 consecutive patients with elevated BST using a standardised questionnaire and compared to a healthy control group. The mean BST value of patients was 18.5±13.0ng/ml (median 15.5ng/ml, range 11.5–103ng/ml), mean age was 52.2±18.4years. The symptoms obtained from the questionnaire are listed in Table 2. Compared with the control group, most symptoms were more common in patients with elevated BST. Cardinal symptoms were muscle and bone ache, vertigo, flush, palpitations, oedema, collapse and nausea (p<0.0001 to p<0.01). Some frequently mentioned symptoms such as debilitating fatigue, headache or pruritus were not discriminative. Although many symptoms occurred more frequently in patients with strongly elevated BST than in patients with slightly elevated BST, these differences were not statistically significant.
Symptoms of 100 patients with elevated basal serum tryptase (BST) and 100 controls. Patients were additionally divided into two groups (slightly elevated BST 11.4–20.0ng/ml and BST>20ng/ml).
| Patients | Controls | |||||
| BST≥11.4ng/ml | BST 11.4–20.0ng/ml | BST >20.0ng/ml | BST≥11.4ng/ml | χ2-Test patients vs. controls | Frequency ratea | |
| Number (male/female) | 100 (32/68) | 81 (26/55) | 19 (6/13) | 0 | ||
| Frequency of symptoms | ||||||
| Fatigue | 56% | 56% | 58% | 37% | <0.01 | 1.5 |
| Meteorismb | 42% | 41% | 47% | 15% | <0.0001 | 2.8 |
| Headache | 37% | 37% | 32% | 38% | n.s. | 1.0 |
| Muscle and bone ache | 36% | 36% | 42% | 16% | <0.001 | 2.3 |
| Swinging mood | 36% | 32% | 47% | 19% | <0.01 | 1.9 |
| Vertigo | 31% | 30% | 37% | 8% | <0.0001 | 3.9 |
| Tachycardia | 29% | 28% | 32% | 10% | <0.001 | 2.9 |
| Flush | 25% | 25% | 26% | 7% | <0.001 | 3.6 |
| Acid reflux | 24% | 25% | 21% | 15% | n.s. | 1.6 |
| Palpitations | 23% | 22% | 26% | 8% | <0.01 | 2.9 |
| Pruritus | 22% | 22% | 21% | 18% | n.s. | 1.2 |
| Diarrhoeab | 22% | 20% | 32% | 8% | <0.01 | 2.7 |
| Hypotension | 18% | 16% | 26% | 7% | <0.05 | 2.6 |
| Abdominal painb | 18% | 16% | 26% | 7% | <0.01 | 2.5 |
| Angio-oedema | 15% | 15% | 16% | 2% | <0.01 | 7.3 |
| Nausea | 14% | 14% | 16% | 4% | <0.05 | 3.5 |
| Urticaria | 10% | 9% | 16% | 4% | n.s. | 2.6 |
| Collapse | 9% | 9% | 11% | 0% | <0.01 | – |
| Ulcer | 6% | 7% | 0% | 0% | <0.05 | – |
| Rash | 5% | 5% | 5% | 3% | n.s. | 1.7 |
| Vomiting | 4% | 4% | 5% | 1% | n.s. | 4.0 |
Out of 75 patients who had been stung by a bee or wasp previously, 18 patients (24%) developed a systemic reaction. Insect venom allergy could be confirmed in 9 out of 15 patients tested (60% – 6× wasp, 1× bee, 2× wasp and bee). In another four patients with questionable positive test results stings had occurred many years ago. In 2/15 patients test results were completely negative. The BST levels of the latter two patients were comparatively low (12.9 and 16.6ng/ml).
68/100 patients had received contrast media at least once and nine (13.2%) reported adverse reactions such as nausea, vertigo, vomiting, chills, dyspnoea, urticaria, diarrhoea, exanthema, restlessness and shock. BST levels in symptomatic and asymptomatic patients were similar (15.2±14.7 vs. 17.1±15.9ng/ml; n.s.).
Adverse reactions after drug intake were reported by 34/100 patients. The most frequent symptoms were cutaneous symptoms, mostly urticaria (n=19) and angio-oedema (n=12). Anaphylactic shock had occurred in four patients and dyspnoea in seven. The suspected responsible drugs were mainly antibiotics (n=18), non-steroidal anti-inflammatory drugs (n=15) and local anaesthetics (n=4). In none of these cases, IgE-mediated type 1 allergy against antibiotics could be confirmed. Patients with severe anaphylactic reactions (grade 3/4) had higher BST levels than patients with less severe reactions. (grade 1: 15.6±2.8ng/ml; grade 2: 16.3±7.7; grade 3+4: 38.7±41.0).
The presence of clonal mast cell disease within this cohort using c-kit mutation analysis and bone marrow biopsy was investigated in only a small minority of the 100 patients. Applying the scoring system of Alvarez-Twose et al.,12 only 15 out of 50 patients with anaphylaxis (30%) showed a score of ≥2 suggestive of clonal mast cell disease. These patients were more often male (p=0.05), more often reacted to Hymenoptera stings (p<0.01), had higher mean BST levels (p=0.05), and more often showed BST values >20ng/ml (p<0.001).
DiscussionMast cell disorders have been recognised as an underlying cause of severe anaphylactic and anaphylactoid reactions. Determination of BST is therefore increasingly used by allergists as a basic screening measure. In this study, we found that 5.9% of patients attending our allergy clinic had an elevated BST level, which is usually regarded as a clear-cut risk factor for anaphylaxis. We confirm this by showing that 21% of patients with elevated BST had a history of anaphylactic reactions, while in those with normal BST it was only 14%. In addition, the severity of anaphylaxis was found to correlate with BST levels, as 2.8% of patients with normal BST, 5.9% of patients with slightly elevated BST and 12.4% of patients with BST levels >20ng/ml experienced severe anaphylactic reactions (grade 3+4). Compared to the reported incidence of anaphylaxis in the general population of 0.3%,13 we estimated an incidence of 0.8% in patients with elevated BST and 0.5% in patients with normal BST, based on an average of 22,500 patients attending our clinic every year. To the best of our knowledge, no comparable data exist on this specific group of patients.
The association between elevated BST and severe anaphylaxis is well documented for Hymenoptera stings.7 Recently, similar observations were also made in children with food allergy.14 Our study confirms these observations, as we found severe reactions in patients with BST levels above 11.4ng/ml 2.7 times more often compared to patients with normal BST (7.3% vs. 2.8%). Published data on the relative importance of drugs, foods and insect venoms as main elicitors of anaphylaxis are controversial.15–16 In our adult study population, insect stings were the most frequent elicitors (62%) followed by drugs (22%) and foods (10%). In contrast, food was the main elicitor in children (50%) followed by insect stings (33%) and drugs (17%), confirming observations by others.13 We also found a higher risk for males and older patients, which again is in accordance with other studies.
One interesting secondary finding of our study is that BST levels in patients with normal tryptase appear to rise with age as already suggested by previous investigators.17 There was a continuous increase in mean BST levels starting at 3.8ng/ml in 20-year-old patients and going up to 5.8ng/in 80-year-old patients. Regression analysis revealed a significant increase in serum tryptase by 0.28ng/ml per decade. Therefore, age should be taken into account when interpreting BST levels even though values above 11.4ng/ml are unlikely due to higher age alone.
A key goal of our study was to define cardinal symptoms associated with elevated BST. Remarkably, we did not observe significant differences in frequency or specificity of symptoms between patients with slightly (<20ng/ml) and strongly elevated BST. All patients with elevated BST suffer from a multitude of common and often unspecific symptoms making discrimination not easy. It is astounding that 56% mention debilitating fatigue, and 37% complain of aching muscles and bones. Although fatigue, aching muscles or swinging mood undoubtedly are highly unspecific symptoms, they were reported significantly more often by patients with elevated BST than by controls. More than this, our control group had been recruited from patients suffering from allergic rhino-conjunctivitis, a disease often associated with day-time fatigue caused by sleep disturbance due to the impairment of nasal breathing. It appears that fatigue itself might be a mast cell-associated symptom as there is no difference in the incidence of, e.g. headaches between patients and controls.
Aching of muscles and bones could possibly be caused by the neurosensitising or vasogenic action of mast cell mediators. Fatigue could also be explained by this pathomechanism.18 Finally, aching of the bones could be associated with osteoporosis, a finding which is relevant in 10–30% of patients with indolent systemic mastocytosis,19 albeit the number of patients with systemic mastocytosis in our study population in unknown. Vertigo was reported by one third of patients, an exceptionally high frequency compared to data reported in the literature on mast cell activation syndromes.12,20 Surprisingly, subjective cardiovascular findings such as tachycardia or palpitations were found in 29% and 23% of patients, respectively.
In our cohort of 100 preselected patients with elevated BST, 34 reported adverse reactions after drug intake, suggesting that drugs are an essential trigger for anaphylactoid reactions. In agreement with this, we found elevated BST in as much as 10% of randomly selected patients with a history of drug intolerance. Likewise, adverse reactions to contrast media were reported by 13% of patients with elevated BST compared to none in the control group, suggesting that assessment of BST is a worthwhile routine measure in the diagnosis of adverse reactions to drugs and contrast media.
Our findings in 248 patients with Hymenoptera venom allergy are in accordance with the literature confirming that elevated BST is a risk factor for severe anaphylaxis after a field sting.7,21 Elevated BST was found in 6% of patients with mean levels being highest in patients with grade 3 and 4 reactions. It is still unclear whether patients suffering from a mast cell-associated disease may develop anaphylactic symptoms after an insect sting even in the absence of venom-specific IgE. While this is suggested by some reports in the literature, other papers showed that using additional diagnostic methods sensitisation can be proven in most patients.22 Hymenoptera venoms contain several peptides capable of destabilising cell membranes and causing non-specific mast cell degranulation, such as mellitin and mast cell degranulating peptide in bee venom, and mastoparan and other kinines in wasp venom. It remains open, however, if the small amount of venom injected during a single sting can release mediators from mast cells in quantities high enough to cause anaphylactic symptoms. 24% of patients with elevated BST in this study reported a systemic sting reaction, but in 40% of them sensitisation could not be proven by routine test procedures. However, as some patients had been stung years ago, these negative results might be also explained by natural loss of hypersensitivity. Taken together, the impact of non-allergic insect sting reactions in patients with mast cell diseases cannot be clarified at this point yet.
In view of a BST level of >20ng/ml as a minor criterion for mastocytosis, more than 75% of raised BST values found in this study were below this cut-off, around 50% even below 15ng/ml. Task groups on MCAS recently proposed to raise the threshold for elevated BST from 11.4 to 15ng/ml,23 which would dramatically reduce the number of “pathologic” tryptase values in our study population by half. Thus, a considerable number of patients presenting with clinical signs of MCAS would neither get a diagnosis nor the recommended antihistamine baseline treatment. In other words, the majority of “elevated” BST values are considered normal by haematologists, whereas for allergists case history is often strongly suggestive of a mast cell-dependent anaphylactic event. Our analysis suggests that patients with moderately elevated BST suffer from a variety of symptoms quite as often as patients with strongly elevated BST, and similarly show a higher risk of anaphylaxis as compared to patients with normal BST. Although proper workup of all patients concerning mastocytosis would be preferable, systematic screening for c-kit mutation and bone marrow biopsy in such a high number of patients is hardly feasible in clinical practice. As an alternative, an increase in serum tryptase of 20%+2ng during a symptomatic episode could serve as an indicator of MCAS, as proposed recently.9 However, it is not just that tryptase levels do not raise during anaphylaxis in 36% of patients,24 serum samples can be rarely obtained during acute reactions in outpatient clinics like ours and hardly ever during milder anaphylactic episodes not requiring medical attendance. Furthermore, saving blood samples for the assessment of tryptase and plasma histamine is not commonly done during emergency treatment.
Alvarez-Twose et al. recently proposed a simple clinical score predicting the likelihood of clonal mast cell disease in patients with anaphylaxis with good sensitivity and specificity.12 Applying this score in case of elevated BST might be a useful measure to identify candidates requiring further diagnostic workup. In our cohort of unselected patients with elevated BST, only 30% of subjects reached a score of +2 points indicative of clonal mast cell disease, thus suggesting that the majority of patients encountered with elevated BST during routine screening probably do not have clonal mast cell disease and would, thus, only unlikely benefit from a burdensome and costly further examination. Likewise, applying the same score to our patients with stinging insect allergy revealed that presumptive clonality is largely constrained to those with severe cardiovascular reactions but less likely in those with mild cutaneous reactions.
Considering the high frequency of elevated BST values in our study population, one may expect elevated BST also in a relevant number of patients eligible to specific immunotherapy against inhalant allergens. Generally spoken, such patients might be considered “at risk” for side effects. Elevated BST was identified as a moderate risk factor for side effects during venom immunotherapy.25–26 In contrast, Asero et al. could not confirm elevated BST as a risk factor in pollen immunotherapy,27 even if due to the small number of patients in this study final conclusions cannot be drawn. In our clinic, we had been recently treating a patient with grass pollen immunotherapy who tolerated the first eight injections without problems, but reacted to the next injection (6.000SQ/U) with severe anaphylaxis (vomiting, collapse, unconsciousness). Blood drawn during the episode showed a histamine level of 27.3ng/ml (normal range <0.30ng/ml) and a strikingly elevated serum tryptase of 732ng/ml. Subsequent inspection of the skin revealed urticaria pigmentosa, and BST assessed some weeks later was 103ng/ml. On the other hand, we are aware of several patients where elevated BST levels were found after finishing three years of uncomplicated immunotherapy. Although routine assessment of BST prior to immunotherapy might be considered beneficial in reducing side effects, a general recommendation cannot be made yet due to a lack of data. Anyhow, in insect venom allergy, elevated BST is a clear-cut indication for specific immunotherapy and current guidelines even recommend life-long therapy.
ConclusionsIn clinical practice it appears reasonable to assess basal serum tryptase not only after anaphylactic reactions, but also in patients suffering from repeated episodes of vertigo, flush, tachycardia, palpitations, angio-oedema and nausea. Even patients with slightly elevated BST have a higher risk of anaphylaxis and experience more severe reactions as compared to patients with normal BST.
Financial supportThe study was self-funded by Floridsdorf Allergy Centre.
Conflicts of interestThe authors have declared no conflicts.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
We thank Ernst Rücklinger (Scientific Consultant/Medical statistics) for statistical analysis.