Allergic rhinitis affects a significant proportion of the European population. Few surveys have investigated this disorder in Greek adults. Our objective was to describe the characteristics of patients with allergic rhinitis in an adult outpatient clinic in Thessaloniki, Greece.
MethodsWe studied the medical records of adult patients referred to a Clinical Immunology outpatient clinic from 2001 to 2007. The diagnostic procedure was not changed during the whole study period, including the same questionnaire used at the time of diagnosis, skin prick tests, and serum specific IgE.
ResultsA total of 1851 patient files with diagnosed allergies were analysed and allergic rhinitis was confirmed in 711 subjects (38.4%). According to ARIA classification, persistent allergic rhinitis was more prevalent than intermittent (54.9% vs. 45.1%), while 60.8% of subjects suffered from moderate/severe disease. In multivariable analysis, factors associated with allergic rhinitis were age (for every 10 years increase, OR: 0.84, 95% CI: 0.77–0.91; p<0.001); working in school environment (teachers or students) (OR: 1.46, 95% CI: 1.05–2.02; p=0.023); parental history of respiratory allergy (OR: 2.41, 95% CI: 1.69–3.43; p<0.001); smoking (OR: 0.71, 95% CI: 0.55–0.91; p=0.007); presence of allergic conjunctivitis (OR: 6.16, 95% CI: 4.71–8.06; p<0.001); and asthma (OR: 2.17, 95% CI: 1.57–3.01; p<0.001). Analysis after multiple imputation corroborated the complete case analysis results.
ConclusionsAllergic rhinitis was documented in 38.4% of studied patients and was frequently characterised by significant morbidity. Factors associated with allergic rhinitis provide insight into the epidemiology of this disorder in our region. Further studies on the general population would contribute to evaluating allergic rhinitis more comprehensively.
Allergic rhinitis (AR) is a very common health problem, affecting up to 25% of the European population.1 Although the evaluation and diagnosis of AR can be achieved in every day practice, it still remains a disease which is often under-diagnosed and under-treated.2,3 Furthermore, the economic impact and the burden on patients’ quality of life are substantial.4
There is no information about AR in Greece in the latest European epidemiological studies,5 whereas specific occupational populations or age groups have been studied so far.6,7
The new ARIA (Allergic Rhinitis and its Impact on Asthma) classification of AR (intermittent or persistent) evaluates the frequency and duration of symptoms. Additionally, the disease can be divided into ‘mild’ or ‘moderate-severe’, according to the severity of symptoms depending on the quality of life of each patient.5
Our study investigates the characteristics of adults with AR who visited an outpatient clinic in Thessaloniki, the second largest city of Greece. The primary objectives were to: (1) determine the prevalence of AR amongst allergic diseases and AR types in an outpatient clinic; (2) describe the factors associated with AR; and (3) investigate the association with other allergic diseases.
Materials and methodsStudy design and participantsThis was a retrospective study with collection of data on successive patients visiting the Clinical Immunology outpatient clinic at a tertiary hospital, AHEPA Hospital in Thessaloniki, from January 2001 until December 2007. Over one million people live in the region of Thessaloniki, which is mainly an urban area.
We retrospectively analysed 2779 medical records and found 1851 eligible files of patients diagnosed with various allergic disorders including AR. Allergic rhinitis was either the main visiting cause, or a co-morbid allergic disease. The criteria of eligibility were: (a) patient-files with allergic disorders; (b) age ≥18 years; (c) recorded data on personal (symptoms) and family history, duration, type and characteristics of manifestations; and (d) documented diagnosis of allergic diseases by means of available methods in this outpatient clinic. Missing information in medical files mainly concerned the occupational environment, the socioeconomic status and characteristics of residence. The diagnostic procedure was unchanged during the whole study period including the same questionnaire used at the time of diagnosis, in vivo, and in vitro tests.
Measures and dataQuestionnairePatients were interviewed by the physicians of the outpatient clinic. The questionnaire was based on the ECRHS II main questionnaire8 and included questions concerning the visit cause, symptoms that could be attributed to allergic reactions, triggering factors causing the symptoms and the characteristics of the reactions (season, place, etc.). Focusing on AR, the subjects were asked about nasal symptoms (sneezing, itchy nose, blocked or runny nose), the duration of symptoms and the association with other atopic disorders such as ocular manifestations, asthma, etc. Patients were also asked about their medical history, atopic family history and demographic details.
In vivo testsSkin prick test (SPT) was performed on the flexor surface of the forearm. A sample of various inhalant allergens (grass [Bermuda, Orchard, Timothy, Rye], cat, dog, Alternaria, Cladosporium, Aspergillus, olive tree, plane-tree, ragweed and mugwort pollen, parietaria (Officinalis/Judaica), house dust Greer/Holister Stier and house dust-mite [Dermatophagoides pteronyssinus/farinae]) (ALLERGOPHARMA kit) were tested. Positive (histamine) and negative (saline) controls were used and results were measured after 15minutes. A wheal of at least 3mm in diameter was defined as a positive SPT result.
In vitro testsThe blood serum was analysed for total IgE concentration and the presence of specific IgE antibodies (sIgE) (Pharmacia®-ImmunoCAP system, Phadia, Uppsala, Sweden) to inhalant allergens. As far as sIgE detection is concerned, a positive result to each allergen was defined only if the concentration of antibodies was higher than 0.35kU/ml.9 The available allergens for sIgE were the same with SPT.
Subjects were considered as suffering from AR when they had positive medical history (data from questionnaire), physical examination, SPT and/or sIgE. It should be mentioned that all studied patients with difficulties in diagnosing had been evaluated by ear/nose/throat (ENT) physicians to exclude AR-like disorders after the first visit. The medical records studied included information which was recorded during the first two visits at the outpatient clinic. Data from questionnaire and physical examination came from the first visit at the outpatient clinic, whereas the results of in vivo/vitro tests and the ENT evaluation were recorded at the second visit. Diagnosis of allergic asthma and conjunctivitis was also confirmed by means of medical history, SPT and/or sIgE. Furthermore, asthmatic patients were evaluated with spirometry and direct airway challenge with inhaled methacholine. The confirmative tests for other allergic diseases were not consistently performed and they were excluded from further analysis.
Statistical analysesThe association between qualitative variables was examined with the chi-square test. Quantitative variables were not normally distributed and the Mann–Whitney U-test was used for their comparison between males and females. Logistic regression analysis was performed and odds ratios were presented with the corresponding 95% confidence intervals (OR, 95% CI). Epidemiological characteristics and other allergic diseases were assessed as predictors of AR in univariate logistic regression models. Those significantly associated with AR (p<0.05) were tested in multivariable logistic regression model by forced entry. Co-linearity was assessed with the tolerance and the variance inflation factor (VIF), while the model fit was evaluated with the Hosmer–Lemeshow test. Discrimination performance of the multivariable model was evaluated with the area under the receiver-operating characteristic curve (AUC). p-Values were two-tailed with a significance level of 0.05. The statistical analyses were performed with SPSS 17.0.
Complete case data were available for 71.2% of patients (1317/1851). Data were missing on residence in 9% of patients and on occupational environment in 20.7% of cases. In order to assess the bias potentially introduced by missing values we performed an additional analysis after multiple imputation of missing values.10 A missing at random pattern of missingness was assumed and the SPSS Missing Values module was used to generate 25 datasets by multiple imputation based on sex, age, personal history of AR/asthma/conjunctivitis, daily exposure to dogs and cats, smoking, parental history of respiratory allergy, living in urban area and occupational environment: house, school, office and countryside. Parameter coefficients derived from combined analysis of the multiple imputed datasets were used to assess the robustness of our findings.
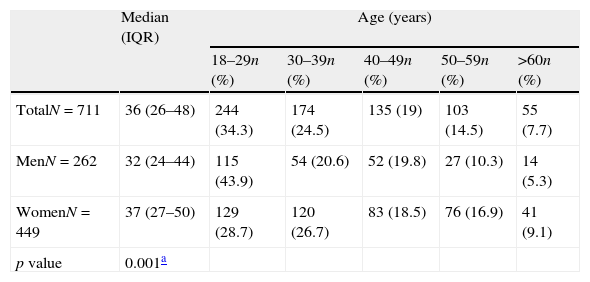
ResultsPrevalenceAR was found in 711 subjects (38.4%) among patients with diagnosed allergies attending the Clinical Immunology outpatient clinic. Male subjects with AR were significantly younger than women (p=0.001) (Table 1).
Distribution of patients with allergic rhinitis by sex and age.
| Median (IQR) | Age (years) | |||||
| 18–29n (%) | 30–39n (%) | 40–49n (%) | 50–59n (%) | >60n (%) | ||
| TotalN=711 | 36 (26–48) | 244 (34.3) | 174 (24.5) | 135 (19) | 103 (14.5) | 55 (7.7) |
| MenN=262 | 32 (24–44) | 115 (43.9) | 54 (20.6) | 52 (19.8) | 27 (10.3) | 14 (5.3) |
| WomenN=449 | 37 (27–50) | 129 (28.7) | 120 (26.7) | 83 (18.5) | 76 (16.9) | 41 (9.1) |
| p value | 0.001a | |||||
Patient age ranged from 18 to 77 years (median 36, Interquartile Range [IQR]: 26, 48; Table 1) and the majority were females (63.2%). Almost three quarters of the patients (72.6%) lived in the area of Thessaloniki, whereas the rest (27.4%) lived in other areas of the region of Central Macedonia, nearby Thessaloniki.
Approximately one-third (n=220, 30.9%) of the patients referred to the outpatient clinic by themselves, another third (n=233, 32.8%) were advised by personal physician (most commonly an ENT physician), whereas the rest (n=258, 36.3%) were informed by other patients or reported no data. Over three quarters of the patients (n=547, 76.9%) claimed AR symptoms as the main visiting cause, whereas the rest (n=164, 23.1%) reported another allergic disorder as their primary problem. A significant number of all patients with AR (596/711, 83.8%) had previously visited either an ENT physician or another expert (allergist, general practitioner, etc.).
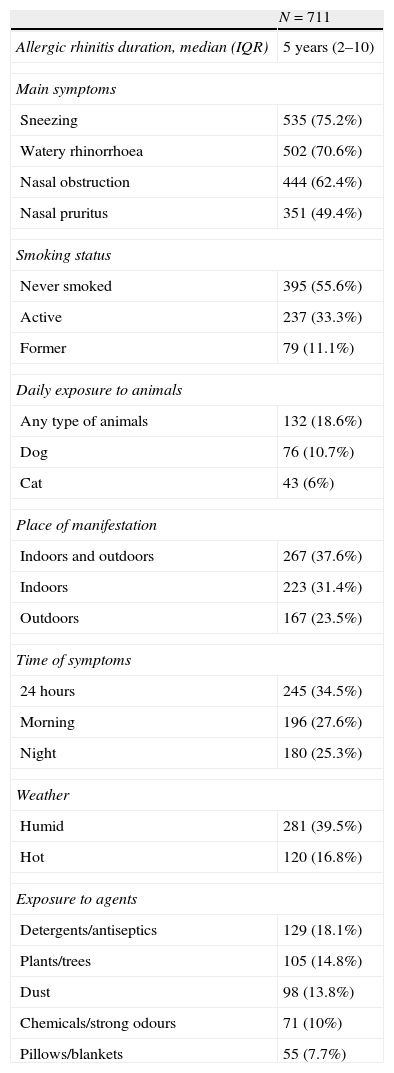
Main clinical characteristics and triggering factors are described in Table 2. In personal history, 603 patients (84.8%) suffered from two or more allergic disorders. The most common co-morbid diseases were allergic conjunctivitis (49.5%), urticaria/angio-oedema (28.1%), asthma (25.2%) and food allergy (16.9%).
Main clinical characteristics and triggering factors associated with symptoms reported by patients.
| N=711 | |
| Allergic rhinitis duration, median (IQR) | 5 years (2–10) |
| Main symptoms | |
| Sneezing | 535 (75.2%) |
| Watery rhinorrhoea | 502 (70.6%) |
| Nasal obstruction | 444 (62.4%) |
| Nasal pruritus | 351 (49.4%) |
| Smoking status | |
| Never smoked | 395 (55.6%) |
| Active | 237 (33.3%) |
| Former | 79 (11.1%) |
| Daily exposure to animals | |
| Any type of animals | 132 (18.6%) |
| Dog | 76 (10.7%) |
| Cat | 43 (6%) |
| Place of manifestation | |
| Indoors and outdoors | 267 (37.6%) |
| Indoors | 223 (31.4%) |
| Outdoors | 167 (23.5%) |
| Time of symptoms | |
| 24 hours | 245 (34.5%) |
| Morning | 196 (27.6%) |
| Night | 180 (25.3%) |
| Weather | |
| Humid | 281 (39.5%) |
| Hot | 120 (16.8%) |
| Exposure to agents | |
| Detergents/antiseptics | 129 (18.1%) |
| Plants/trees | 105 (14.8%) |
| Dust | 98 (13.8%) |
| Chemicals/strong odours | 71 (10%) |
| Pillows/blankets | 55 (7.7%) |
A total of 309 (43.5%) subjects were under medication for atopic disorders including AR when visiting the outpatient clinic. The most frequently reported drugs were intranasal decongestants (36.7%), intranasal corticosteroids (32.9%), topical eye medication (24.9%), oral antihistamines (22.1%) and inhalant corticosteroids (13.9%).
Allergy diagnosis and responsible allergensSkin prick tests and sIgE confirmed the diagnosis of AR. Skin prick tests were carried out in 565 patients (79.5%) and sIgE in 496 (69.8%). All subjects had positive results either to SPTs or to sIgE, whereas the most frequent allergens both in SPTs and sIgE were grass, house dust-mite, olive tree (Olea europaea), parietaria (Officinalis/Judaica) and moulds (Alternaria, Cladosporium, Aspergillus, Candida albicans). The majority of patients (61.1%) reacted to more than two allergens in SPTs.
Total serum IgE concentration (median 90IU/ml, IQR: 32–233) was measured in 487 patients (68.5%).
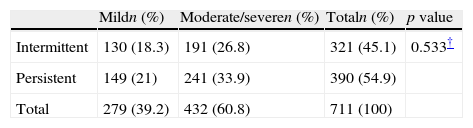
AR classificationThe AR classification according to ARIA criteria is presented in Table 3. Persistent AR was diagnosed in over half of the subjects (54.9%), whereas 60.8% of the patients suffered from moderate or severe disease. Comparing intermittent and persistent type with age and sex showed no statistical significance (p=0.974 and p=0.911, respectively).
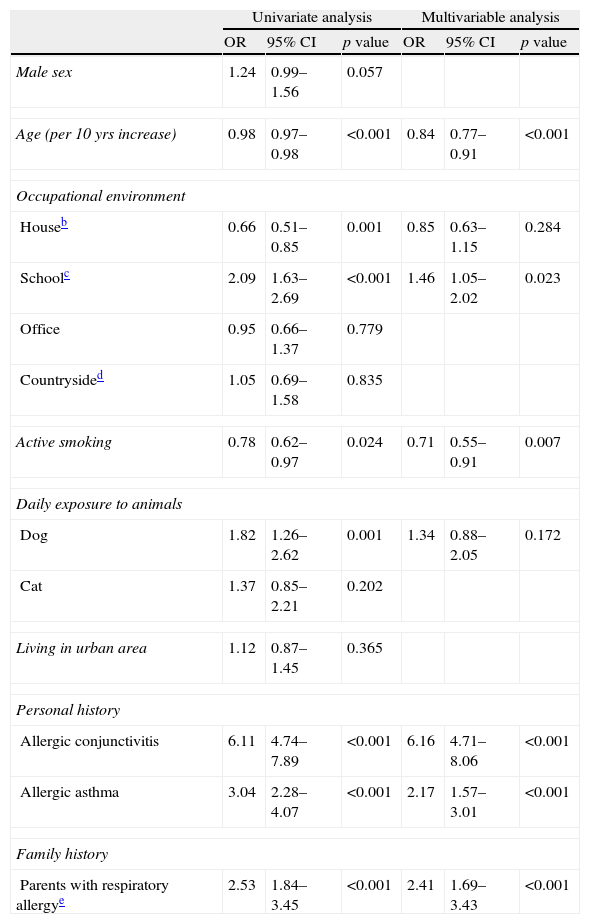
Factors associated with ARSubjects with parental history of respiratory allergy (OR: 2.41, 95% CI: 1.69–3.43; p<0.001), working in school environment (OR: 1.46, 95% CI: 1.05–2.02; p=0.023), having allergic conjunctivitis (OR: 6.16, 95% CI: 4.71–8.06; p<0.001), and asthma (OR: 2.17, 95% CI: 1.57–3.01; p<0.001) were found to have an increased risk of suffering from AR. Conversely, active smoking was associated with lower odds of AR (OR: 0.71, 95% CI: 0.55–0.91; p=0.007). Furthermore, for every 10 years increase in patient age, the odds of AR decreased by 16% (p<0.001, Table 4). There was no co-linearity present as assessed by tolerance and variance inflation factor (VIF), for tolerance the lowest value was 0.75 while for VIF the highest value was 1.34. The Hosmer and Lemeshow test indicated a good fit of the data (p=0.504) and the discriminative performance of the model was modest (AUC=0.771, 95% CI: 0.746–0.796). Analysis after multiple imputation corroborated the complete case analysis results (Table 4).
Factors associated with allergic rhinitis in studied patients with data available on all variables.a
| Univariate analysis | Multivariable analysis | |||||
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Male sex | 1.24 | 0.99–1.56 | 0.057 | |||
| Age (per 10 yrs increase) | 0.98 | 0.97–0.98 | <0.001 | 0.84 | 0.77–0.91 | <0.001 |
| Occupational environment | ||||||
| Houseb | 0.66 | 0.51–0.85 | 0.001 | 0.85 | 0.63–1.15 | 0.284 |
| Schoolc | 2.09 | 1.63–2.69 | <0.001 | 1.46 | 1.05–2.02 | 0.023 |
| Office | 0.95 | 0.66–1.37 | 0.779 | |||
| Countrysided | 1.05 | 0.69–1.58 | 0.835 | |||
| Active smoking | 0.78 | 0.62–0.97 | 0.024 | 0.71 | 0.55–0.91 | 0.007 |
| Daily exposure to animals | ||||||
| Dog | 1.82 | 1.26–2.62 | 0.001 | 1.34 | 0.88–2.05 | 0.172 |
| Cat | 1.37 | 0.85–2.21 | 0.202 | |||
| Living in urban area | 1.12 | 0.87–1.45 | 0.365 | |||
| Personal history | ||||||
| Allergic conjunctivitis | 6.11 | 4.74–7.89 | <0.001 | 6.16 | 4.71–8.06 | <0.001 |
| Allergic asthma | 3.04 | 2.28–4.07 | <0.001 | 2.17 | 1.57–3.01 | <0.001 |
| Family history | ||||||
| Parents with respiratory allergye | 2.53 | 1.84–3.45 | <0.001 | 2.41 | 1.69–3.43 | <0.001 |
In this study, AR occurred in over a third of patients (38.4%) that referred to a Clinical Immunology outpatient clinic in Thessaloniki (Greece) and were diagnosed with allergic disorders. Most epidemiological studies that have been carried out in the general population have found that AR is the commonest allergic disease, affecting 10–40% of people worldwide.11
Our results showed that AR patients were mainly young persons and there was a female predominance, which is also described by other authors.12,13 The fact that young adults (18–29 years old) more frequently suffered from AR is also indicated in other studies.14,15
The high percentage of patients who visited the outpatient clinic mainly for AR (76.9%) and had previously visited an ENT physician (83.8%) shows the urgent need for subjects to be treated and relieved of the troublesome symptoms. The need to expand and improve primary healthcare in Greece is illustrated by the fact that the majority of patients who attended the outpatient clinic were not referred by their personal/family doctor.
The rates of AR symptoms in this study are similar to the findings of other surveys.12,16,17 Allergic rhinitis co-morbidity with other atopic manifestations concerned over a quarter of patients (25.2%) and almost half of AR subjects (49.5%) who also suffered from asthma and conjunctivitis, respectively. The percentage of patients suffering from AR and asthma in our study is not different from the international range (10–40%).18–21 The prevalence of co-morbidity between AR and conjunctivitis is similar to another study from Greece,22 higher than a population-based study in USA23 and lower than other relevant studies.12,13,16
Despite the fact that the majority of patients had previously sought medical care, only 43.5% of them were under medication for AR, asthma and conjunctivitis. This finding could be attributed to poor adherence to prescribed medication.
The diagnostic procedure and determination of AR in this study was based on positive SPTs and sIgE results. These methods are easy and safe to perform, especially SPTs which are available in any expert clinical setting. Positive results mostly concerned grass, house dust-mite and olive tree pollen. The allergic sensitisation profile in this study is comparable with findings in recent Mediterranean studies, carried out in Greece,22 Spain,24,25 Italy26,27 and Portugal.28 Most of our results are in agreement with recent reports on Greek adult population.29
The predominance of persistent type (54.9%) in our findings is similar to results of other Mediterranean studies22,24,25 but there are also studies which showed intermittent AR as the more frequent type.30,31 These results indicate the substantial differences in the prevalence of AR types, which are probably attributed to the different characteristics of the populations. A significant number of subjects (60.8%) suffered from moderate or severe symptoms. The remarkable severity of AR in our study is comparable with findings of other authors.22,24,31 Patients with the more severe and troublesome manifestations are anticipated to visit and seek for help in outpatient clinics of tertiary hospitals.
Multivariable analysis showed that parental history of respiratory allergy and working in school environment were positively related to AR in studied patients. The role of family history has been described by other authors,17 especially concerning AR in children. The association between AR and working in school environment could be attributed to the fact that subjects working in school environment could more easily visit the outpatient clinic due to their public insurance. Although schools may be a significant site of exposure to indoor allergens, there is not enough published data on the contribution of this exposure to allergic sensitisation and the development of allergic disorders.32 The negative association between AR and active smoking in our results is in agreement with another study which considers smoking as an unclear factor in causing AR or even as a potential low risk factor.33 The presence of allergic conjunctivitis and asthma increased the likelihood of studied subjects to suffer from AR. The strong association of AR and asthma is well studied and assessed by the ARIA initiative in order to study and control AR impact on asthma.18 Allergic rhinitis is also strongly associated with allergic conjunctivitis and these disorders are often referred to as allergic rhinoconjunctivitis.34
There are a number of limitations in our study. First, the study population involves selected patients of an outpatient clinic and, thus, our estimates do not apply to the general population.
Second, the majority of patients who visited the outpatient clinic came from urban areas and there is not adequate representation of the rural population. Furthermore, the presence of other expert outpatient clinics in Thessaloniki could lead to selection bias in the representative collection of subjects.
Third, data were collected from a questionnaire which was created by the physicians of the outpatient clinic taking into account the recent nomenclature for allergies and the ECRHS II main questionnaire. Although missing data were observed, we performed multiple imputation to assess the robustness of the complete case analysis. It should also be mentioned that different physicians interviewed the patients and completed the questionnaires which is prone to measurement bias.
In conclusion, we investigated the characteristics of adults with AR who attended a clinical immunology outpatient clinic in Thessaloniki, Greece over a 7-year period. Allergic rhinitis is a common disease which is associated with significant morbidity and substantially affects the quality of life of 60.8% of AR patients in our study population. Our report conforming to the ARIA classification enables comparisons with other European surveys and could serve as reference for future studies in Greece. Further studies could prospectively evaluate the epidemiology of AR in the general population. Concerted action should be undertaken to diagnose and control AR from a public health standpoint.
Conflicts of interestThe authors have no conflict of interest to declare.
The authors are grateful to the Scientific Council of AHEPA Hospital for providing the archives of the Clinical Immunology outpatient clinic.
Dr. Theodore Alexandropoulos was supported with a scholarship from the State Scholarships Foundation of Greece (I.K.Y.).