It is thought that airway inflammation is more common in obese asthmatic patients because inflammation is harder to control and does not respond well to glucocorticoid treatment.
ObjectiveThis study's aim was to investigate the effect of obesity on airway and systemic inflammation in children with asthma and to identify the biomarkers that play a role in this inflammation.
MethodsThe study included patients aged 6–16 years who were diagnosed with asthma in the paediatric allergy outpatient clinic of Bagcilar Training and Research Hospital in Turkey. Complete blood count parameters were compared between three groups: obese asthmatic (n=43), obese non-asthmatic (n=45), and non-obese non-asthmatic (control group, n=30). Levels of high-sensitive CRP (hs-CRP), neutrophil gelatinase-associated lipocalin (NGAL), osteopontin (OPN), and matrix metalloproteinase-9 (MMP-9), and 25(OH)-vitamin D were compared between the groups.
ResultsNo statistically significant differences were observed in 25(OH)-vitamin D, NGAL, OPN, hs-CRP, and MMP-9 levels between groups. There was a statistically significant negative correlation between FEV1/FVC and NGAL and MMP-9.
ConclusionThis is the first study to investigate levels of hs-CRP, NGAL, OPN, MMP-9, and 25(OH)-vitamin D in obese asthmatic children. Larger studies with sputum and BAL examinations are required to determine the potential of biomarkers for identifying inflammation in obese asthmatic children.
Asthma is a heterogenic, chronic inflammatory disease characterised by reversible obstruction and hypersensitivity of the airways. Recent studies have shown a clear correlation between the prevalence of obesity and asthma, but the connection between the two remains unexplained.1,2 Studies on the relationship between obesity and asthma have emphasised the mechanic, airway, and systemic inflammatory factors.2 Obesity changes pulmonary physiology and lung mechanics, which lead to a decrease in lung volume.3 Other studies have shown that systemic inflammation in overweight and obese individuals can trigger asthma.3–6 It is thought that airway inflammation is more common in obese asthmatic patients since inflammation is harder to control in obese and asthmatic patients and does not respond well to glucocorticoid treatment.4 Pro-inflammatory adipokines and proteins released from the adipose tissue in obese individuals are the reasons for this. It is thought that these adipokines contribute to asthma and the hypersensitivity of the airways by inducing airway inflammation or increasing existing inflammation.5–10
For these reasons, it is crucial to identify the biomarkers that play a role in inflammation in order to improve diagnosis and treatment in obese asthmatic cases. Identifying specific non-invasive biomarkers is even more important in children. Most of the current diagnostic methods cannot be used efficiently with children since they require the cooperation of the patient and some such methods are invasive.11,12 Osteopontin (OPN) is produced in different types of cells in the immune system. It has been demonstrated that T-cells, B-cells, macrophages, neutrophils, eosinophils, natural killer cells, CD11c-positive dendritic cells (DCs), and bronchial epithelium, in particular, produce the protein OPN. OPN is expressed during inflammation and is related to Th2 lymphocyte activity. Previous studies have shown the role of OPN in asthma, allergic rhinitis, allergic conjunctivitis, and responses to venom immunotherapy.7 Neutrophil gelatinase-associated lipocalin (NGAL) is a 25kDa glycoprotein which was recently shown to be a matrix protein of specific granules of human neutrophils.10 NGAL is secreted by neutrophils and other cells such as respiratory and intestinal epithelial cells, vascular endothelial cells, adipose tissue, macrophages, and tubuli cells in the kidneys.10 Extracellular matrix proteins play a role in airway remodelling and matrix metalloproteinase-9 (MMP-9) and NGAL are associated with lung capacity and clinical severity, in particular.9,10 An increase in the levels of MMP-9 and NGAL in bronchoalveolar lavage (BAL) fluid samples, possibly caused by structural changes in the airways, was evident in a number of studies on patients with asthma, pulmonary emphysema, and chronic obstructive pulmonary disease (COPD).10 However, there is no study on the role of OPN and NGAL in obese asthmatic paediatric patients.
This study's aim was to investigate the effect of obesity on airway and systemic inflammation in children with asthma and to identify the biomarkers that play a role in this inflammation.
Materials and methodsPatient populationAll patients were 6–16 years old and observed in the paediatric allergy outpatient clinic of the Bagcilar Training and Research Hospital. Those diagnosed as obese and asthmatic between April and August 2015 were included in this study. The diagnosis and treatment followed the guidelines of the Global Initiative for Asthma (GINA).13 Skin prick tests were performed on all patients for the same allergens. Body mass index (BMI) percentages were based on age, sex, height, and weight, following Neyzi et al.’s study of Turkish children aged 2–18.14 Patients with a BMI at the 95% percentile or greater were considered obese and those with a lower BMI were considered non-obese. The control group comprised non-obese, non-asthmatic children in the same age range who had no allergies or symptoms of infection. They were admitted to the same paediatric outpatient clinic for general medical examinations. The three study groups were: obese asthmatic (Group I, n=43), obese non-asthmatic (Group II, n=45), and the control group, non-obese non-asthmatic (Group III, n=30). Group I was given GINA classifications based on control tests, and spirometric measurements were taken. Venous blood samples were taken from all groups in order to compare levels of high-sensitive CRP (hs-CRP), NGAL, OPN, MMP-9, and 25(OH)-vitamin D. Possible correlations were explored between the spirometric measurements in group I, inflammation markers, and 25(OH)-vitamin D levels.
Patients were excluded if they had experienced worsening asthma or taken systemic steroids within one month of the study, or if they had had an acute or chronic infection or other systemic disease such as hepatic, renal, cardiovascular diseases, diabetes mellitus, cancer, sepsis, or systemic inflammatory disorders. Informed consent was given by all patients’ parents and was approved by the Research Ethics Committee of Bagcilar Training and Research Hospital (approval number 2015/403). The study is compatible with all ethical issues of the Helsinki declaration.
Counting blood samplesTo determine OPN levels, peripheral venous blood samples were collected after patients fasted overnight for 10h. Samples were centrifuged at 3000rpm for 10min within 1h of collection. Isolated plasma samples were kept at −80°C until the assay. The micro Elisa method was used for the analysis of serum OPN concentration with micro-ELISA kits purchased from the company Affymetrix eBioscience. To detect the levels of 25(OH)-vitamin D, the Electroluminescence Immunoassay (ECLIA) method was employed using a Roche-Cobas 6000 immunoassay analyser. MMP-9, hs-CRP, and NGAL tests were conducted following the micro-ELISA method using commercial micro-ELISA kits purchased from the Sunred Biological Technology of Shanghai, China. All micro-ELISA measurements were performed at a wavelength of 450nm and in a DAR800 micro-ELISA reader. An intra-assay coefficient of variation of the ELISA system was 3–9% and its inter-assay coefficient of variation was 4–10.2%.
SpirometryIn the spirometry laboratory, spirometry was applied to groups I and II a minimum of three times using a ZAN 100 (Germany). Bronchodilator reversibility was determined to be more than 12%, or 200ml change from the FEV1 baseline. The measurement of these parameters was performed according to GINA criteria.13
Skin prick testSkin prick tests were performed on the anterior surface of the forearm when patients were not under the effect of antihistamines. Skin prick tests were performed using a Stallerpoint device to test for common aeroallergens (Dermatophagoides pteronyssinus, Dermatophagoides farinae), a mixture of grass pollens (Lolium perenne, Dactylis glomerata, Phleum pratense, Anthoxanthum odoratum, Poa pratensis, Festuca elatior, Agrostis vulgaris, Holcus lanatus, Cynodon dactylon, Avena sativa, Avena fatua, Lotus Corniculatus), a mixture of grain pollens (oats, wheat, barley, corn), a mixture of tree pollens (Acer pseudoplatanus, Aesculus hippocastanum, Robinia pseudoacacia, Tilia platyphyllos, Platanus vulgaris), weed-mix pollens (Medicago sativa, Trifolium pratense, Brassica nigra, Urtica dioica, Rumex acetosa), Alternaria alternaria, cockroaches (Blattella germanica), and cat and dog dander (Stallergenes SA, 92160 Antony, France). Histamine (10mg/ml) and physiological saline were used as positive and negative controls, respectively. Skin reactions were evaluated after 20min. A positive reaction was characterised as wheal diameter ≥3mm. Atopy was classified with at least one positive reaction in the skin test.
Assessment of symptom controlWe assessed the children's level of asthma symptom control using the GINA guidelines. We used three categories: well controlled, partly controlled, and uncontrolled.13
Statistical analysisStatistical analyses were conducted with the 2007 program Number Cruncher Statistical System (NCSS) (Utah, USA). Besides the descriptive statistics (average, standard deviation), unidirectional variation analysis was used in multiple group comparisons of normally distributed variables. Tukey's multiple comparison tests were used in subgroup comparisons, independent t-test were used in the paired-group comparisons, Kruskal–Wallis tests were used in multiple-group comparisons of variables not distributed normally, Dunn's multiple comparison tests were used in subgroup comparisons, and chi-square tests were used to compare qualitative data. The results were evaluated at the significance level of p<0.05.
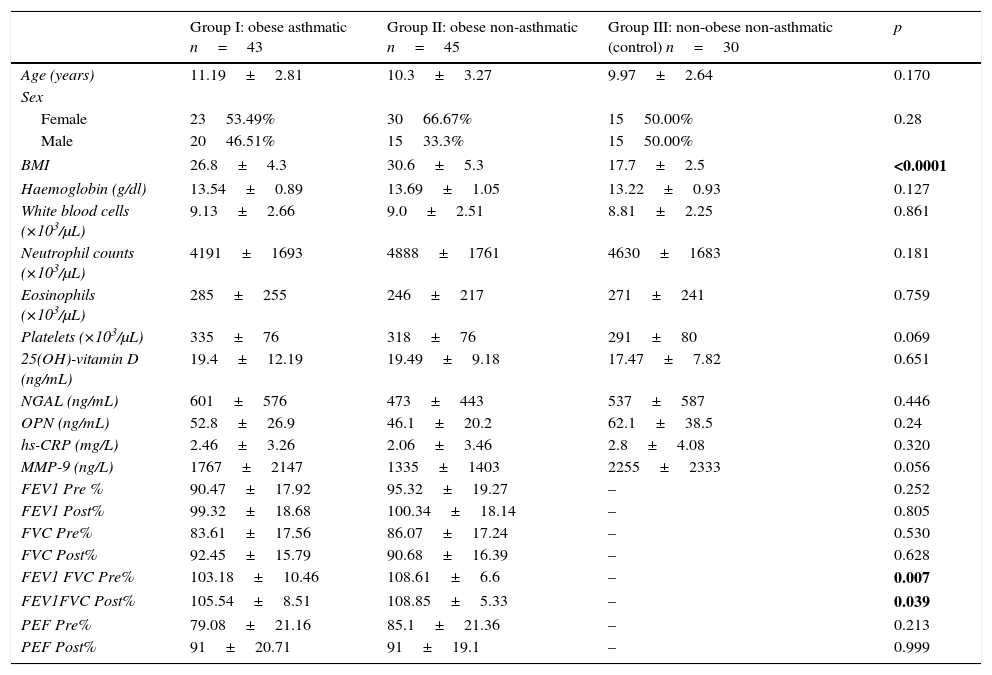
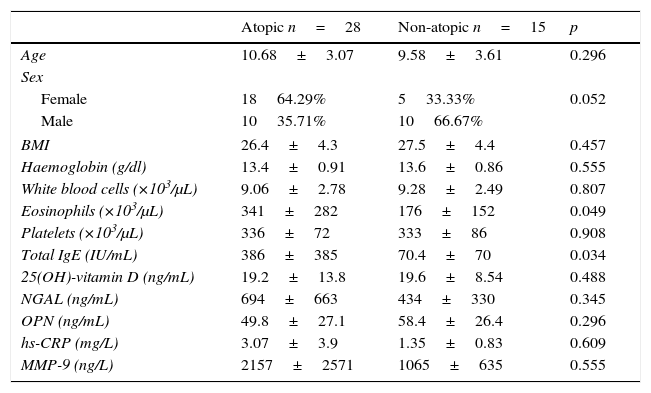
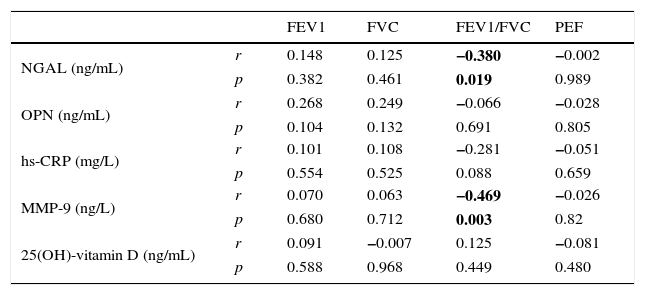
ResultsThe average age of the obese asthmatic patients (group I) was 11.19±2.81 years; 53.4% of them were girls, 65% of them were atopic, and they were all sensitive to house dust mites. In the spirometric examination of group I, the average forced expiratory volume in one second (FEV1) was 90.4±17.9, the forced vital capacity (FVC) average was 99.3±18.6, the FEV1/FVC average was 103.1±10.4, and the peak expiratory flow (PEF) average was 79.0±21.1. In GINA's 2015 terms, 34.9% (n=15) of obese asthmatic patients were receiving second step treatment and 65.1% (n=28) were receiving third step treatment. No statistically significant differences were observed in the average ages or sexes between any of the three groups (p>0.05) (Table 1). No statistically significant differences were observed between groups’ hemogram parameters or the group averages of 25(OH)-vitamin D, NGAL, OPN, hs-CRP, or MMP-9 levels (p>0.05) (Table 1). These parameters were not significantly different between subgroups with and without atopy in group I (p>0.05). Eosinophil counts and total IgE levels were significantly higher in atopic patients (Table 2). In terms of the potential correlation between spirometric measurements and markers in group I, there was no statistically significant differences among FEV1, PEF, and FVC measurements and levels of NGAL, OPN, hs-CRP, MMP-9, or 25(OH)-vitamin D (p>0.05). However, there was a statistically significant negative correlation between FEV1/FVC values and NGAL and MMP-9 values (p=0.019 and p=0.003, respectively) (Table 3).
Data and comparisons for the three study groups.
| Group I: obese asthmatic n=43 | Group II: obese non-asthmatic n=45 | Group III: non-obese non-asthmatic (control) n=30 | p | |
|---|---|---|---|---|
| Age (years) | 11.19±2.81 | 10.3±3.27 | 9.97±2.64 | 0.170 |
| Sex | ||||
| Female | 2353.49% | 3066.67% | 1550.00% | 0.28 |
| Male | 2046.51% | 1533.3% | 1550.00% | |
| BMI | 26.8±4.3 | 30.6±5.3 | 17.7±2.5 | <0.0001 |
| Haemoglobin (g/dl) | 13.54±0.89 | 13.69±1.05 | 13.22±0.93 | 0.127 |
| White blood cells (×103/μL) | 9.13±2.66 | 9.0±2.51 | 8.81±2.25 | 0.861 |
| Neutrophil counts (×103/μL) | 4191±1693 | 4888±1761 | 4630±1683 | 0.181 |
| Eosinophils (×103/μL) | 285±255 | 246±217 | 271±241 | 0.759 |
| Platelets (×103/μL) | 335±76 | 318±76 | 291±80 | 0.069 |
| 25(OH)-vitamin D (ng/mL) | 19.4±12.19 | 19.49±9.18 | 17.47±7.82 | 0.651 |
| NGAL (ng/mL) | 601±576 | 473±443 | 537±587 | 0.446 |
| OPN (ng/mL) | 52.8±26.9 | 46.1±20.2 | 62.1±38.5 | 0.24 |
| hs-CRP (mg/L) | 2.46±3.26 | 2.06±3.46 | 2.8±4.08 | 0.320 |
| MMP-9 (ng/L) | 1767±2147 | 1335±1403 | 2255±2333 | 0.056 |
| FEV1 Pre % | 90.47±17.92 | 95.32±19.27 | – | 0.252 |
| FEV1 Post% | 99.32±18.68 | 100.34±18.14 | – | 0.805 |
| FVC Pre% | 83.61±17.56 | 86.07±17.24 | – | 0.530 |
| FVC Post% | 92.45±15.79 | 90.68±16.39 | – | 0.628 |
| FEV1 FVC Pre% | 103.18±10.46 | 108.61±6.6 | – | 0.007 |
| FEV1FVC Post% | 105.54±8.51 | 108.85±5.33 | – | 0.039 |
| PEF Pre% | 79.08±21.16 | 85.1±21.36 | – | 0.213 |
| PEF Post% | 91±20.71 | 91±19.1 | – | 0.999 |
Bold values indicate statistically significance at P<0.05.
Data and comparisons for atopic and non-atopic patients in group I (obese asthmatic).
| Atopic n=28 | Non-atopic n=15 | p | |
|---|---|---|---|
| Age | 10.68±3.07 | 9.58±3.61 | 0.296 |
| Sex | |||
| Female | 1864.29% | 533.33% | 0.052 |
| Male | 1035.71% | 1066.67% | |
| BMI | 26.4±4.3 | 27.5±4.4 | 0.457 |
| Haemoglobin (g/dl) | 13.4±0.91 | 13.6±0.86 | 0.555 |
| White blood cells (×103/μL) | 9.06±2.78 | 9.28±2.49 | 0.807 |
| Eosinophils (×103/μL) | 341±282 | 176±152 | 0.049 |
| Platelets (×103/μL) | 336±72 | 333±86 | 0.908 |
| Total IgE (IU/mL) | 386±385 | 70.4±70 | 0.034 |
| 25(OH)-vitamin D (ng/mL) | 19.2±13.8 | 19.6±8.54 | 0.488 |
| NGAL (ng/mL) | 694±663 | 434±330 | 0.345 |
| OPN (ng/mL) | 49.8±27.1 | 58.4±26.4 | 0.296 |
| hs-CRP (mg/L) | 3.07±3.9 | 1.35±0.83 | 0.609 |
| MMP-9 (ng/L) | 2157±2571 | 1065±635 | 0.555 |
Correlations between markers and spirometric measurements in group I (obese asthmatic).
| FEV1 | FVC | FEV1/FVC | PEF | ||
|---|---|---|---|---|---|
| NGAL (ng/mL) | r | 0.148 | 0.125 | −0.380 | −0.002 |
| p | 0.382 | 0.461 | 0.019 | 0.989 | |
| OPN (ng/mL) | r | 0.268 | 0.249 | −0.066 | −0.028 |
| p | 0.104 | 0.132 | 0.691 | 0.805 | |
| hs-CRP (mg/L) | r | 0.101 | 0.108 | −0.281 | −0.051 |
| p | 0.554 | 0.525 | 0.088 | 0.659 | |
| MMP-9 (ng/L) | r | 0.070 | 0.063 | −0.469 | −0.026 |
| p | 0.680 | 0.712 | 0.003 | 0.82 | |
| 25(OH)-vitamin D (ng/mL) | r | 0.091 | −0.007 | 0.125 | −0.081 |
| p | 0.588 | 0.968 | 0.449 | 0.480 |
Bold values indicate statistically significance at P<0.05.
All three groups (obese asthmatic, obese non-asthmatic, and control) had similar levels of the biomarkers OPN, NGAL, MMP-9, and hs-CRP.
Other studies have shown that OPN has a role in Th2-mediated inflammation, but its role in childhood obese-asthma is not exactly known.8 Samitas et al.15 found that the amount of OPN and BAL liquid of stable adult patients was higher than the control group. However, when examined during inflammation, serum levels were low. Liu et al.16 reported increased OPN levels in patients with allergic rhinitis, which were positively correlated with total nasal symptom scoring, total eosinophil count, serum Eosinophil Cationic Protein (ECP), and IL-5. In other studies on adults with asthma, it was found that OPN levels of serum, saliva, and BAL liquid samples were high.17,18 In the study conducted by Zülfikar et al.,8 OPN levels in asthmatic children over age five were higher than those of the control group. A subgroup analysis showed that levels in children under the age of five were similar to the control group. They explained that this was because they were waiting for temporary wheezing attacks and that the increase in OPN levels was due to Th2-mediated inflammation in patients under the age of five. Our study is the first to focus on the relationship of childhood obese-asthma and OPN. OPN serum levels were similar in all three groups and there was no difference between atopic and non-atopic patients. This may be because the study did not have patients with severe asthma and the measurements were taken when asthma symptoms were under control.
High-sensitive CRP is a good marker of low-level inflammation. In a study on patients with severe asthma, hs-CRP levels in obese patients was found to be higher.19 In another study on adults, hs-CRP levels were higher in obese patients than non-obese patients and higher in patients with asthma than those without it.20 In another study on adults, hs-CRP levels were higher in obese patients than non-obese patients, higher in patients with asthma than those without it, and higher in obese asthmatic patients than obese non-asthmatic patients.21 Studies on young adults showed that hs-CRP levels were inversely correlated with respiratory function test parameters and that this relationship was independent of asthma and obesity.22,23 Moreover, there was no correlation between spirometry measurements and hs-CRP levels. Jensen et al.24 examined groups of obese and non-obese asthmatic patients, similar to our study, and also found no between-group differences in hs-CRP levels in children. In our study, no difference was detected in hs-CRP levels between all three groups (obese asthmatic, non-obese asthmatic, and non-obese non-asthmatic). The differences between studies suggest that the relationship between hs-CRP levels, obesity, and asthma is a complicated interaction affected by multiple parameters.
It has been suggested that asthma symptoms in the obese-asthma phenotype could be connected to lower vitamin D levels in obese individuals.25,26 Menon et al.27 found no difference in vitamin D levels between asthmatic and non-asthmatic patients. However, they did find that vitamin D levels were lower in obese asthmatic and obese patients than non-obese asthmatic patients and the control group. In our study, there was no difference in 25(OH)-vitamin D levels between all three groups and there was no relationship between BMI and 25(OH)-vitamin D. These results agree with another similar study in Turkey, in which there were no differences in 25(OH)-vitamin D levels between four groups: obese-asthmatic, obese non-asthmatic, non-obese asthmatic, and non-obese non-asthmatic.28
NGAL is a specific granule of human neutrophils that is secreted by a number of tissues such as the respiratory epithelial cells and macrophages. NGAL plays a role in diseases such as acute kidney injury, obesity, and cancer.10 Increased NGAL and MMP-9 levels in asthmatic patients with structural airway changes have been reported.10,29,30 However, the relationship between NGAL, MMP-9, and disease severity is not known for obese children with asthma. There is only one study on NGAL in these children, which reported that Transforming Growth Factor Beta 1 (TGFB1) could reflect airway remodelling but NGAL could not predict it.10 Ohbayashi et al.31 reported increased MMP-9 levels in bronchoalveolar lavage, sputum, and serum samples of patients with asthma and increased MMP-9 immune reactivity in bronchial biopsy samples. In our study, although NGAL and MMP-9 levels were similar to the control group, a statistically significant negative correlation was detected between the rates of FEV1/FVC and the levels of NGAL and MMP-9 in spirometric measurements of obese asthmatic patients. These results made us think that NGAL could be a potential marker for airway inflammation in obese asthmatic cases, however, there is a need for studies with larger samples and patients with severe asthma. Since it is known that MMP-9 levels increase in asthmatic patients, there is a need for studies that include non-obese asthmatic patients in order to assess the potential of MMP-9 as a biomarker in obese asthmatic paediatric cases.
The limitations of our study are the following: a small sample of patients, examining inflammatory biomarkers when symptoms were under control, not including non-obese asthmatic patients, not including severe obese asthmatic patients, not doing sputum cytology or bronchoalveolar lavage examinations, and not assessing fractional exhaled nitric oxide (FeNO) levels, which may have revealed the effect of obesity on airway inflammation. There are very few studies that have investigated the effect of obesity on airway inflammation during childhood.24 As far as we know, this is the first study to test hs-CRP, NGAL, OPN, MMP-9, and 25(OH)-vitamin D levels in obese asthmatic children. Since the relationship between obesity and asthma is a complicated interaction affected by multiple parameters, clarifying it will require large-scale studies that evaluate clinical symptoms with sputum and BAL examinations. Such studies would be able to better define the role of biomarkers in obese asthmatic children and their potential as biomarkers.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Trial registrationNot applicable.
Funding sourceNo external funding was secured for this study.
Authors’ contributionNacaroglu, Hikmet Tekin: literature search, study design, data collection, manuscript preparation/editing, final manuscript approval.
Bostan Gayret, Özlem: literature search, study design, data collection, manuscript preparation/editing, final manuscript approval.
Erol, Meltem: literature search, data collection, data analysis, data interpretation.
Büke, Övgü: literature search, data collection, data analysis, data interpretation.
Zengi, Oguzhan: literature search, manuscript preparation/editing, data analysis.
Tasdemir, Mehmet: literature search, study design, final manuscript approval.
Tasdemir, Zeynep: literature search, manuscript preparation/editing, data analysis.
Yigit, Özgül: literature search, manuscript preparation/editing, data analysis.
Conflict of interestThe authors have no conflicts of interest to disclose.
We thank the Bagcilar training and research hospital. This study was reviewed and approved by the review board of Bagcilar training and research hospital.