OM-85 is an immunostimulant bacterial lysate, which has been proven effective in reducing the number of lower airways infections. We investigated the efficacy of the bacterial lysate OM-85 in the primary prevention of a murine model of asthma.
MethodsIn the first phase of our study the animals received doses of 0.5μg, 5μg and 50μg of OM-85 through gavage for five days (days −10 to −6 of the protocol), 10 days prior to starting the sensitisation with ovalbumin (OVA), in order to evaluate the results of dose–response protocols. A single dose (5μg) was then chosen in order to verify in detail the effect of OM-85 on the pulmonary allergic response. Total/differential cells count and cytokine levels (IL-4, IL-5, IL-13 and IFN-γ) from bronchoalveolar lavage fluid (BALF), OVA-specific IgE levels from serum, lung function and lung histopathological analysis were evaluated.
ResultsOM-85 did not reduce pulmonary eosinophilic response, regardless of the dose used. In the phase protocol using 5μg/animal of OM-85, no difference was shown among the groups studied, including total cell and eosinophil counts in BALF, serum OVA-specific IgE, lung histopathologic findings and lung resistance. However, OM-85 decreased IL-5 and IL-13 levels in BALF.
ConclusionsOM-85, administered in early life in mice in human-equivalent doses, does not inhibit the development of allergic pulmonary response in mice.
Asthma is a chronic inflammatory disease of the lower airways that affects nearly 300 million people worldwide, with a high prevalence in children (10–30% of cases), characterised by chronic airway inflammation and bronchial hyper-responsiveness, resulting in recurrent respiratory symptoms.1 The use of continuous inhaled corticosteroids is the preferred therapy for the control of asthma in all ages. However, corticosteroids are associated with local and systemic adverse events and are only effective when the disease is already established. Hence, the search for effective primary prevention of asthma has been directed to early life approaches. Probiotics,2–4 prevention of viral infections,5,6 and environmental exposure to bacterial products such as lipopolysaccharide (LPS)7 appear to reduce the risk of developing asthma.
OM-85 is a bacterial-derived orally-delivered immunostimulant that boost systemic mucosal immune defences. Composed of a mix of acidic proteins, peptides and amino acids, with smaller components of lipopolysaccharides and lipoteichoic acid, extracted from eight common pathogenic bacteria of the upper respiratory tract (Haemophilus influenzae, Streptococcus pneumoniae, Klebsiella pneumoniae, Klebsiella ozaenae, Staphylococcus aureus, Streptococcus pyogenes, Streptococcus viridans, and Neisseria catarrhalis), OM-85 has been used worldwide for over 20 years to reduce the number of acute respiratory infections in adults and children,8,9 with immunomodulatory activities.10 One Cochrane review of 35 placebo-controlled studies showed that 40% of patients younger than 18 years of age who had used immunostimulants reduced the number of infections in the lower airways compared to placebo groups.11 Other studies have shown that OM-85 reduced the number of wheezing episodes in children (1–6 years).9,12 These findings, along with the concept of “immune system's common mucosa”,13 in which mucosal antigen exposure leads to stimulation of an immune response in a distant mucosa, generate an attractive hypothesis that oral administration of OM-85 in early life inhibits the development of allergic asthma. In this context, Navarro et al., using a murine model of Th2 response induced by ovalbumin (OVA), demonstrated that the use of OM-85 inhibited bronchial hyperresponsiveness, lung inflammation, production of specific IgE, airway mucus secretion and eosinophilia, reducing the Th2 immune response, and increasing the number of Treg cells in the intestinal and airway mucosa.14 Strickland et al. have also shown that OM-85 stimulates regulatory T cell (Treg) cells in distant tissues, with inhibition of allergic pulmonary response in mice.10 However, the doses used in both studies could be considered extremely high in a translational perspective, comparing with human-equivalent doses.15 The aim of our study is to specifically assess the efficacy of OM-85 in preventing airway inflammation in a murine model of pulmonary eosinophilic response, performing a first phase using dose–response protocols, compatible with human-equivalent doses, and subsequently performing a more detailed efficacy test, using only one chosen dosage.
MethodsAnimalsWe used 42 female BALB/c mice, between 3 and 4 weeks of life (from CeMBE, PUCRS). The animals were fed with balanced rodent chow and water ad libitum. Animals were kept in cages, under 12 light hours and 12 dark hours (6:00/18:00).
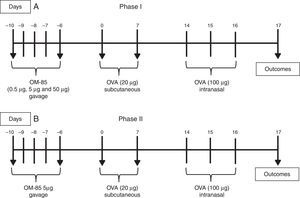
Protocol of asthma and OM-85 dose–response curveThe dose used for ovalbumin (OVA) sensitisation was 20μg, subcutaneously (SC), diluted in 200μL of Dulbecco's phosphate buffered saline (DPBS) for all groups. Intranasal challenge (IN) was performed with a dose of 100μg of OVA, diluted in 50μL of DPBS. To allow pulmonary aspiration, animals were anaesthetised in a chamber using isoflurane for 30s. For the Phase I, animals were divided into three experimental groups according to the dose of bacterial lysate (OM-85) received. The animals received doses of 0.5μg, 5μg and 50μg of OM-85 (Paxoral®, Farmasa, Brazil) by gavage, once a day, for five days (Day −10 to −6 protocol), 10 days before beginning OVA sensitisation, which occurred on days 0 and 7 of the protocol. The doses of OM-85 used in the present study compared to previous studies and package leaflet of the commercially available OM-85 (Broncho-vaxom capsules, Takeda Pharma, Japan) are presented in Table 1. The dose of the package leaflet of OM-85, for a child with 30kg, is between the two higher doses we have used (5 and 50μg). All doses were calculated based on human-equivalent dose formula.15 We have also chosen lower doses to test a dose–response curve because higher doses used in other studies would require larger volumes for gavage in mice, which is not feasible in murine protocols.10,14 A control group received only DPBS in the same volume as the animals treated with OM-85. The intranasal challenge with OVA was performed for three consecutive days, on days 14, 15 and 16, after sensitisation. The protocol is presented in Fig. 1A.
Doses of OM-85 considering human-equivalent doses used in our protocol, when compared to previous studies, and to the package leaflet commercially available.
| Study dose | Dose (mg/kg) | Human-equivalent dose (mg/kg) |
|---|---|---|
| Present study | ||
| 0.5μg | 0.02 | 0.002 |
| 5μg | 0.2 | 0.02 |
| 50μg | 2 | 0.2 |
| Package leaflet (child with 30kg) | 0.97 | 0.12 |
| Navarro et al. 14 | 1000 | 120 |
| Strickland et al. 10 | 400 | 96a |
From the results of the dose–response protocols, the dose of 5μg of OM-85, as justified above, was chosen in order to verify in detail its effect on the inhibition of pulmonary allergic response (Phase II). The experiment was then repeated, with OVA, OVA-treated OM-85 (5μg), and DPBS group. Sensitisation and challenge with OVA were performed in the same manner as Phase I. The protocol of Phase II is illustrated in Fig. 1B.
Bronchoalveolar lavage fluid (BALF)BALF was performed on both phases of the study by cannulation of the trachea with a blunt needle, after anaesthesia with ketamine (0.4mg/g) and xylazine (0.2mg/g). A solution of DPBS (1mL) was instilled intratracheally and aspirated after two consecutive instillations. After the procedure, animals were euthanised with lethal doses of ketamine and xylazine (three-fold dose) and disposed in accordance with the rules of the institution.
Total and differential cells count in BALFBALF samples were centrifuged at 2000rpm for 4min. The precipitate was resuspended in 350μL of DPBS. Total cells count (CTC) and cell viability were performed in all samples through the method of exclusion of trypan blue in a Neubauer chamber (BOECO, Germany). For the differential cells count, 80μL of the precipitate suspension was cytocentrifuged (30g for 5min) and allowed to air-dry at room temperature. For the differential cell analysis, the BALF suspension was determined by cytospin preparations stained with Panótico Rápido (Laborclin®, Brazil). Cell types were observed under optical microscopy and expressed as percentage, after counting 400 cells.
Flow cytometryBALF supernatants were frozen at −80°C for further analysis. IL-4, IL-5, IL-13 and IFN-γ levels were then measured through flow cytometry (FACSCanto II, BD Biosciences, USA) using Cytometric Bead Array (CBA) Set Flex Kit, according to the manufacturer's instructions (BD Biosciences, USA). The limit of detection for IL-4 assay is 0.3pg/mL, for IL-5 is 0.9pg/mL and for IL-13 is 2.4pg/mL and for IFN-γ is 1.5pg/mL.
OVA-specific IgESensitisation to OVA was analysed by measuring OVA-specific IgE in BALF through ELISA (Mdbioproducts®) according to the manufacturer's instructions.
Lung functionPulmonary function was performed only in Phase II, after cannulation of the trachea by tracheotomy, with the anaesthetised animal. The animal was maintained on a ventilator (Harvard Minivent Hugo Sachs Elektronik, USA) for 5min before the test began. Six technical measures of forced oscillation during a pause in the ventilator (6s) were performed. Data was collected and analysed using specific software (WinLung, Hungary) with measurements of airway resistance (Raw) through the impedance method.16
Histopathological analysisAt the end of the protocol, lungs were removed in Phase II, with perfusion performed with 10% buffered formalin by gravity column (20mmHg). Slides were prepared from 4μm lung tissue paraffin blocks sections and stained with Haematoxylin and Eosin (H&E) for histopathological analysis. Assessment of qualitative bronchial/peribronchial inflammatory abnormalities was reported.
Statistical analysisResults were analysed using Statistical Package for the Social Sciences, version 20.0 (SPSS Inc.). Student's t test was performed in order to compare groups, and p≤0.05 was considered statistically significant. Data are presented as mean±SD.
EthicsThe study was conducted under ethical standards for research in animal models, following the recommendations of the Brazilian Society of Laboratory Animal Science (SBCAL), advocating the use of fewer animals and adequate management of pain and suffering during the study procedures and euthanasia. Our study was approved by the Ethics Committee for the Use of Animals (CEUA) of our institution (09/00106).
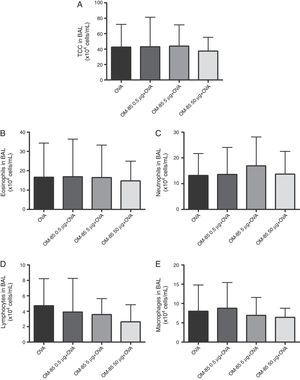
ResultsWe used OM-85 in early life to evaluate the effect on pulmonary eosinophilic inhibition in BALB/c mice. Initially, we conducted a first phase in order to attempt to choose a more effective dose of OM-85 in the inhibition of the pulmonary allergic response in our protocol. Total cells count from BALF were not different between the OVA group, 0.5μg, 5μg and 50μg groups (p>0.05). The number of eosinophils (p>0.05), lymphocytes (p>0.05), macrophages (p>0.05), and neutrophils (p>0.05) from BALF were also not significantly different between the groups studied. Inflammatory cell numbers from BALF in the groups studied in Phase I are shown in Fig. 2.
Total cells count (A) and differential cells count: Eosinophils (B), neutrophils (C), lymphocytes (D) and macrophages (E) in BALF of mice in Phase I of the study. Data are expressed as mean±SD (4–8 animals in each group). Student's t test was used. OVA: ovalbumin; DPBS: phosphate-buffered saline; BALF: bronchoalveolar lavage fluid.
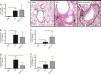
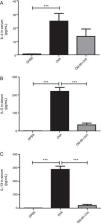
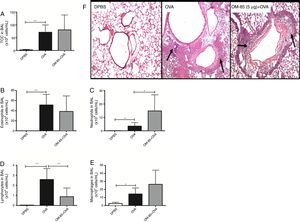
We verified in Phase I that OM-85 did not reduce the pulmonary eosinophilic response when compared to the OVA group, regardless of the dose used. We then decided to choose the intermediate dose used (5μg/animal) for Phase II. The experiment was then repeated with a more detailed analysis (expanding the outcomes analysed), comparing with OVA and DPBS groups, in order to assess the lack of effect of early administration of OM-85 on the inhibition of the eosinophilic response in the lungs of mice. In Phase II, total cells count, eosinophils, and macrophages were not significantly different between OM-85+OVA and OVA group (Fig. 3A, B and E, respectively). In this phase, neutrophils were significantly higher in OM-85+OVA group (p<0.05) and lymphocytes (p<0.01) were significantly lower when compared to the OVA group (Fig. 3C and D, respectively). In this context, histopathological analysis between all OVA sensitised and challenged groups, including OM-85 group, presented an intense peribronchovascular cell infiltrate. The DPBS group had preserved anatomy with no inflammatory changes (Fig. 3F).
Total cells count (A) and differential cells count: Eosinophils (B), neutrophils (C), lymphocytes (D) and macrophages (E) in BALF of mice in Phase II of the study. Data are expressed as mean±SD for 4–8 animals in each group. Different from OVA group: *p<0.05; **p<0.01. Student's t test was used. OVA: ovalbumin; DPBS: phosphate-buffered saline; BALF: bronchoalveolar lavage fluid. Representative photomicrographs of stained sections (H&E) of lung histology (A–C, 200×) in (F). Arrows indicate inflammatory cells infiltration.
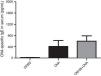
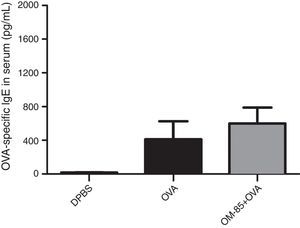
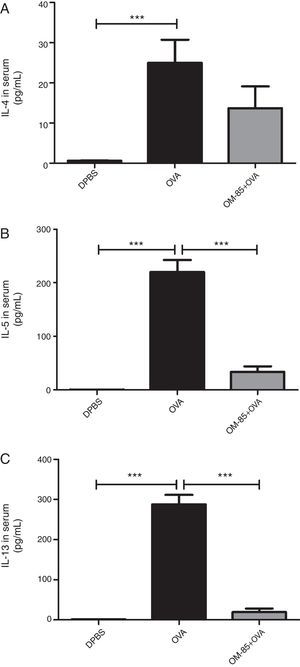
Moreover, we evaluated the effect of OM-85 (5μg/animal) on OVA-specific IgE in serum (Fig. 4) and cytokine levels (IL-4, IL-5, IL-13 and IFN-γ) in BALF (Fig. 5). We did not observe any alteration in OVA-specific IgE levels between the groups. Fig. 5 shows that OM-85 significantly decreased the levels of IL-5 (p<0.001) and IL-13 (p<0.001) in BALF when compared to the OVA group, but we did not observe differences between the groups in IL-4 levels (p>0.05). The levels of IFN-γ in BALF were all below the detection levels (data not shown).
IL-4 (A), IL-5 (B), IL-13 (C) and IFN-γ (D) cytokines levels in BALF. Data are expressed as mean±SD (4–8 animals in each group). Different from OVA group, ***p<0.001. Student's t test was used. OVA: ovalbumin; DPBS: phosphate-buffered saline; BALF: bronchoalveolar lavage fluid; IFN-γ: interferon-γ.
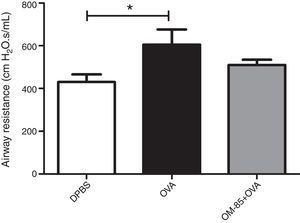
Finally, airway resistance using forced oscillation method showed a difference between OVA and the DPBS group (p<0.05). In addition, we did not observe any other difference in OM-85+OVA (p>0.05) compared to the OVA group (Fig. 6).
DiscussionWe showed that OM-85 administered in early life, in human-equivalent doses, did not inhibit the development of allergic pulmonary response in mice, when total cells count, eosinophil numbers, IL-4 and OVA-specific IgE levels were analysed. We tested three doses of OM-85, with human-equivalent doses, extending the analysis of outcomes using the intermediate dose tested (Phase II). In Phase II, we have again found no difference in the allergic pulmonary response in OM-85-treated mice.
Over the last two decades, studies have reported that OM-85 activates macrophages, natural killer cells and increase cytokine production against infections.17–21 In this context, OM-85 has been shown to reduce respiratory tract infections in children and adults.20,22–25 Particularly in asthma, there is a lack of human studies. Two previous studies evaluated the effect of OM-85 on the pulmonary allergic response in rodents, analysing the mechanistic aspect of Treg cells stimulation with OM-85 administered through the gastrointestinal tract.10,14 Strickland et al. showed that OM-85 inhibited bronchial hyperresponsiveness, lung inflammation, and the production of specific IgE and Th2 cytokines, in a rat OVA model of asthma. This study has also shown that after aeroallergen inhalation and before the airways translocation of the allergen via the regional draining lymph nodes, the quiescent dendritic cells activation of the airway mucosa (CDMVA) occurs through interaction with memory T cells, leading to increasing CD86 expression and increased T cell stimulatory activity through these dendritic cells in situ. Subsequent interactions between CDMVA and transient memory T cells result in local activation of Th cells, with determined duration of Treg cell activity. The importance of Treg response in the control of bronchial hyperresponsiveness and allergic response may result in a new target of treatment for asthma.10 Navarro et al. (2011), in the same line of rationale, had shown that OM-85 also inhibited pulmonary allergic response in mice, stimulating Treg cells production in the lung.14 However, our study showed conflicting results. OM-85 did not inhibit the allergic pulmonary responses in a murine model of asthma, using three OM-85 doses. The most likely explanation for our findings is that we used lower OM-85 doses, which were based on human-equivalent dose calculation and the package leaflet of a commercially available OM-85. If we consider the conversion of the dose used in animal studies of Strickland et al. (2011) and Navarro et al. (2011), the human equivalent dose of OM-85 for children (considering a child weighing 30kg) would be converted to a daily dose of 2880mg and 3600mg, respectively. These doses would be extremely high for use in children, making translational possibilities unlikely.10,14 The doses of 5μg and 50μg that we used of OM-85 in mice would allow us to conduct clinical trials in children, because these doses would result in human-equivalent doses of 0.02mg/kg and 0.2mg/kg, respectively. However, our doses showed no inhibitory effect on pulmonary allergic response. Furthermore, we previously tested a dose of 700μg (human-equivalent dose of 2.85mg/kg) of OM-85 on a pilot study in the same model, also with no inhibitory pulmonary allergic effect (unpublished data).
In Phase II of our study, we found changes in BALF neutrophils and lymphocytes. We could not find an explanation for these findings, and we believe that they do not interfere with the main conclusion of our study in relation to the lack of an inhibitory effect of OM-85 in the allergic pulmonary response in mice. In BALF, IL-4 levels were not different between the OVA and OM-85 groups, and IFN-γ was not detected in any group. IFN-γ is usually systemically high in mice treated with OM-85,8 but Navarro et al. (2011) found the same results of our study, suggesting that OM-85 may not stimulate a Th1 response in the lungs. Our negative findings of OM-85 in this asthma model are also further reinforced by the similar OVA-specific IgE in BALF between the OVA and the OM-85 groups. On the other hand, we found that OM-85 decreased IL-5 and IL-13 levels. In this context, Han et al. (2014) demonstrated that low doses of OM-85, orally-administered for three months before sensitisation, inhibited Th2 response by reducing cytokines (IL-4, IL-5, or IL-13) in the nasal lavage fluids.26 In addition, the airway inflammation, hyperresponsiveness and variable airflow obstruction are mediated, at least in part, by Th2 cells and associated cytokines (IL-4, IL-5 and IL-13), which are increased in BALF, sputum and bronchial biopsies of asthmatics patients.27
Our study has some limitations. We did not assess bronchial hyperresponsiveness to methacholine and did not explore in more detail other mechanistic factors, such as Treg expression in mice treated with OM-85. However, as we show that OM-85 did not inhibit eosinophilic response in the lungs in our murine preclinical study, with three different human-equivalent doses, in the authors’ opinion, these limitations become less important. In OVA mice models, the most important outcome is the inflammatory eosinophilic response in the lungs in order to assess efficacy of new therapies for asthma.
In conclusion, our study showed that the early use of OM-85 in human-equivalent doses does not inhibit the development of asthma in a mice model. Safety pre-clinical studies with high doses of OM-85, with further clinical studies, and the effect of OM-85 on viral infections in children with atopic backgrounds are still issues to be studied and explored.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Financial supportThis study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq, Brazil.
Author's contributionAll authors must approve the final version of the manuscript.
Andrea Rodrigues: data generation; analysis and interpretation of the data and preparation or critical revision of the manuscript.
Lucien Perondi Gualdi: analysis and interpretation of the data and preparation or critical revision of the manuscript.
Rodrigo Godinho de Souza: data generation; analysis and interpretation of the data.
Mauro Henrique Moraes Vargas: data generation; analysis and interpretation of the data.
Nailê Karine Nuñez: data generation; analysis and interpretation of the data.
Aline Andrea da Cunha: data generation; analysis and interpretation of the data and preparation or critical revision of the manuscript.
Marcus Herbert Jones: conception and design of the study.
Leonardo Araújo Pinto: conception and design of the study.
Renato Tetelbom Stein: conception and design of the study.
Paulo Márcio Pitrez: conception and design of the study and preparation or critical revision of the manuscript.
Conflict of interestThe authors have no conflict of interest to declare.