Exposure to pets can be a predisposing factor in the development of certain diseases, including allergic diseases.
ObjectiveWe analyzed the role that exposure to indoor dogs and cats plays in the prevalence of allergic diseases.
MethodsWe examined the cross-sectional data of 1056 women and 936 men aged 15 to 18 years; these individuals were selected through stratified and cluster random sampling. We asked all participants about their exposure to indoor dogs and cats during the year that preceded our study. The prevalence of allergic diseases was determined through core questions taken from The International Study of Asthma and Allergies in Childhood questionnaire.
ResultsThe prevalence was 12.7% (95% CI: 11.3%–14.2%) for asthma, 9.0% (95% CI: 7.8%–10.4%) for allergic rhinitis, and 5.2% (95% CI: 4.3%–6.2%) for atopic dermatitis. The multivariate analyses showed that exposure to indoor dogs, but not indoor cats, was associated with asthma prevalence (aOR 1.37; 95% CI: 1.03–1.83), as was male sex (aOR=1.42; 95% CI: 1.08–1.86), a personal history of allergic rhinitis (aOR=3.24; 95% CI: 2.25–4.66), and a maternal history of asthma (aOR=3.06; 95% CI: 1.89–4.98). The population attributable risk for exposure to indoor dogs was 18%. Notably, neither allergic rhinitis nor atopic dermatitis was found to be associated with dog or cat exposure (p> 0.05).
ConclusionExposure to dogs in late adolescence is a factor associated with asthma, although its contribution to the development of asthma should be investigated in new studies.
For millennia, both dogs and cats have accompanied humans throughout life’s journeys. Overall, pets offer diverse benefits to human health and quality of life1,2; however, this is not always the case. Exposure to pets may be a predisposing factor for the development of certain diseases, including allergic diseases.
Currently, multiple observational studies have evaluated the effect that exposure to dogs and cats has on the development of allergic diseases, and the results have been contradictory. One of the most thorough studies addressing this issue is the International Study of Asthma and Allergies in Childhood Phase Three, which noted that early exposure to cats was a risk factor for asthma, rhinoconjunctivitis, and atopic dermatitis in children aged 6–7 years.3 In contrast, a meta-analysis showed that early exposure to dogs and cats neither increased nor decreased the risk of developing asthma or allergic rhinitis during childhood.4 Lastly, another study reported that among children aged 4–6, exposure to dogs and cats was negatively associated with the prevalence of asthma and allergic rhinitis; however, when exposure occurred at an early age, its association was positive.5
Hereditary factors have been consistently tied to allergic diseases. For example, in our country, it has been reported that a maternal history of asthma increases the risk of asthma in children; furthermore, a personal history of allergic rhinitis increases the risk of asthma development almost fivefold.6 With regard to allergic rhinitis, there is an association with a family history of allergic disease, while atopic dermatitis has been linked to a paternal history of the disease.7 However, knowing whether exposure to dogs or cats plays an independent role in the development of allergic diseases would be very useful for public health.
Because exposure to dogs and cats may be favorable or unfavorable, depending on the circumstances in which an individual is exposed to a pet, the objective of our study was to determine whether exposure to dogs or cats plays a role independent of hereditary factors or family atopy in the prevalence of asthma, allergic rhinitis and atopic dermatitis, among a sample of late adolescents.
MethodsEthicsThe adolescents in our investigation had to give verbal consent to be included in this project; the decision of non-participation was respected when it was requested by the subjects of the study. The Ethics and Research Committees of Guadalajara’s Nuevo Hospital Civil approved this study.
Design and subjectsIn a cross-sectional population-based study, we included 1992 students from private and public high schools within the city of Guadalajara, Mexico; all participants were between the ages of 15 and 18 years. The corresponding data were compiled from April to June 2016.
Sample and sampling techniqueThe methodology has been explained previously in more detail8; briefly, Guadalajara is a city located in Western Mexico, and the city is divided into seven administrative districts (strata). We took a subsample from each of these districts through stratified random sampling. We then randomly selected at least one school (defined as a cluster) for each stratum; again, each grade level was considered a new stratum, and then we randomly selected a group from these strata. Lastly, each student from the selected group was given a sequential number, and through random selection, we obtained our sample.
QuestionnaireThe prevalence of allergic diseases, such as asthma, allergic rhinitis and atopic dermatitis, was determined based on the responses to the questions provided by The International Study of Asthma and Allergies in Childhood9; Have you ever been diagnosed with asthma? Have you ever been diagnosed with allergic rhinitis? Have you ever been diagnosed with atopic dermatitis or atopic neurodermatitis or eczema? All affirmative responses were divided by the total number of surveyed students.
All data were gathered through a structured questionnaire in which we asked about exposure to indoor dogs and cats during the year that preceded our survey (In the past 12 months, have you been exposed to dogs indoors? In the past 12 months, have you been exposed to cats indoors?). We also asked about each individual’s sex, age, parental history of allergic diseases, and current tobacco intake (Do you currently smoke?), and exercise routine (Do you participate in physical activities such as walking, running, swimming, gymnastics, or ball games for at least 30min?). The questionnaire was answered directly by each of the participating adolescents.
Statistical analysesCategorical variables were compared using the chi-square test or Fisher’s exact-test, and continuous variables with a normal distribution were compared using Student’s t test. For the calculation of the prevalence of allergic diseases, the number of subjects with an affirmative answer was divided by the total number of subjects surveyed (those with missing answers were excluded from the denominator); this proportion was expressed as a percentage together with its respective 95% confidence interval (95% CI) for proportions. In contrast, a multivariate analysis was performed for each of the allergic diseases: asthma, allergic rhinitis and atopic dermatitis (dependent variables). Within the group of covariates, in addition to the main independent variable (exposure to dogs), the sex variable and all of the variables that showed significant association in the univariate analysis were included as possible confounders. Due to the sample size of the subjects selected, a model could be constructed with seven to nine covariates. In all of the models, the independent covariates introduced were sex, exposure to indoor dogs, personal history of other allergic diseases different from the dependent variable, maternal history of asthma, maternal history of allergic rhinitis, maternal history of atopic dermatitis, paternal history of asthma and paternal history of allergic rhinitis. In these models, binary logistic regression was used to calculate odds ratios and confidence intervals; variables were excluded in the adjusted model when p was greater than 0.05 using the forward conditional method. Population attributable risk for exposure to indoor dogs was calculated as OR–1/OR multiplied by the proportion of subjects with asthma exposed to pets. Data were analyzed using IBM® SPSS® Statistics 20 (IBM Corp., Armonk, NY, USA) for Windows.
ResultsWe included 1992 students (53.0% were male, and 47.0% were female), the response rate was 98.1%; among these students, the disease prevalence was as follows: asthma, 12.7% (95% CI: 11.3%–14.2%); allergic rhinitis, 9.0% (95% CI: 7.8%–10.4%); and atopic dermatitis, 5.2% (95% CI: 4.3%–6.2%).
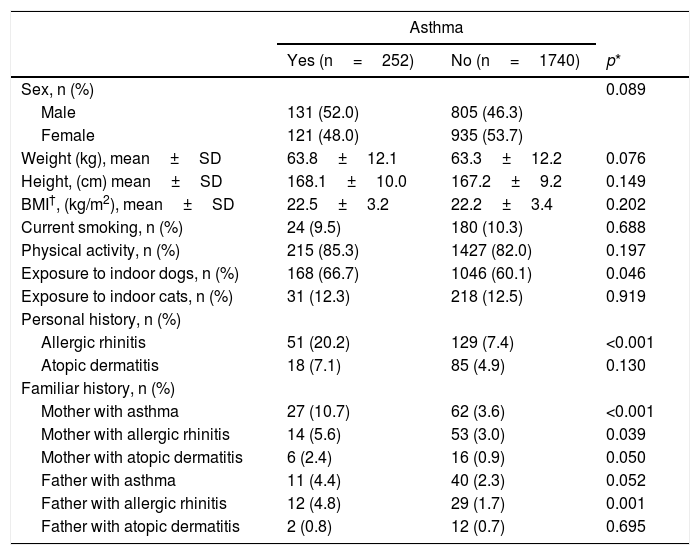
In comparison to students that did not have asthma, adolescents with asthma had a significantly higher exposure to indoor dogs during the year that preceded our survey (p=0.046); In contrast, we did not find statistically significant differences between groups in exposure to cats (Table 1). Furthermore, a personal history of allergic rhinitis was more prevalent in students with asthma (20.2% vs. 7.4%, p<0.001). A maternal history of asthma and allergic rhinitis, as well as a paternal history of allergic rhinitis, was found to be associated with the prevalence of asthma.
Characteristics of the asthmatic population.
| Asthma | |||
|---|---|---|---|
| Yes (n=252) | No (n=1740) | p* | |
| Sex, n (%) | 0.089 | ||
| Male | 131 (52.0) | 805 (46.3) | |
| Female | 121 (48.0) | 935 (53.7) | |
| Weight (kg), mean±SD | 63.8±12.1 | 63.3±12.2 | 0.076 |
| Height, (cm) mean±SD | 168.1±10.0 | 167.2±9.2 | 0.149 |
| BMI†, (kg/m2), mean±SD | 22.5±3.2 | 22.2±3.4 | 0.202 |
| Current smoking, n (%) | 24 (9.5) | 180 (10.3) | 0.688 |
| Physical activity, n (%) | 215 (85.3) | 1427 (82.0) | 0.197 |
| Exposure to indoor dogs, n (%) | 168 (66.7) | 1046 (60.1) | 0.046 |
| Exposure to indoor cats, n (%) | 31 (12.3) | 218 (12.5) | 0.919 |
| Personal history, n (%) | |||
| Allergic rhinitis | 51 (20.2) | 129 (7.4) | <0.001 |
| Atopic dermatitis | 18 (7.1) | 85 (4.9) | 0.130 |
| Familiar history, n (%) | |||
| Mother with asthma | 27 (10.7) | 62 (3.6) | <0.001 |
| Mother with allergic rhinitis | 14 (5.6) | 53 (3.0) | 0.039 |
| Mother with atopic dermatitis | 6 (2.4) | 16 (0.9) | 0.050 |
| Father with asthma | 11 (4.4) | 40 (2.3) | 0.052 |
| Father with allergic rhinitis | 12 (4.8) | 29 (1.7) | 0.001 |
| Father with atopic dermatitis | 2 (0.8) | 12 (0.7) | 0.695 |
SD: Standard deviation.
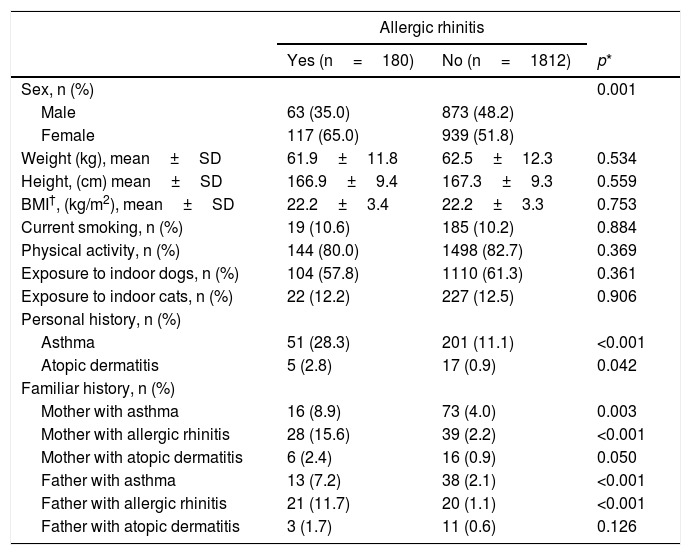
With regard to allergic rhinitis, this disease was more prevalent among women (Table 2). In this case, exposure to dogs and cats was not linked to its frequency. A personal history of asthma or atopic dermatitis was significantly associated with allergic rhinitis. Additionally, a maternal or paternal history of asthma or allergic rhinitis was also linked to the prevalence of allergic rhinitis.
Characteristics of the population with allergic rhinitis.
| Allergic rhinitis | |||
|---|---|---|---|
| Yes (n=180) | No (n=1812) | p* | |
| Sex, n (%) | 0.001 | ||
| Male | 63 (35.0) | 873 (48.2) | |
| Female | 117 (65.0) | 939 (51.8) | |
| Weight (kg), mean±SD | 61.9±11.8 | 62.5±12.3 | 0.534 |
| Height, (cm) mean±SD | 166.9±9.4 | 167.3±9.3 | 0.559 |
| BMI†, (kg/m2), mean±SD | 22.2±3.4 | 22.2±3.3 | 0.753 |
| Current smoking, n (%) | 19 (10.6) | 185 (10.2) | 0.884 |
| Physical activity, n (%) | 144 (80.0) | 1498 (82.7) | 0.369 |
| Exposure to indoor dogs, n (%) | 104 (57.8) | 1110 (61.3) | 0.361 |
| Exposure to indoor cats, n (%) | 22 (12.2) | 227 (12.5) | 0.906 |
| Personal history, n (%) | |||
| Asthma | 51 (28.3) | 201 (11.1) | <0.001 |
| Atopic dermatitis | 5 (2.8) | 17 (0.9) | 0.042 |
| Familiar history, n (%) | |||
| Mother with asthma | 16 (8.9) | 73 (4.0) | 0.003 |
| Mother with allergic rhinitis | 28 (15.6) | 39 (2.2) | <0.001 |
| Mother with atopic dermatitis | 6 (2.4) | 16 (0.9) | 0.050 |
| Father with asthma | 13 (7.2) | 38 (2.1) | <0.001 |
| Father with allergic rhinitis | 21 (11.7) | 20 (1.1) | <0.001 |
| Father with atopic dermatitis | 3 (1.7) | 11 (0.6) | 0.126 |
SD: Standard deviation.
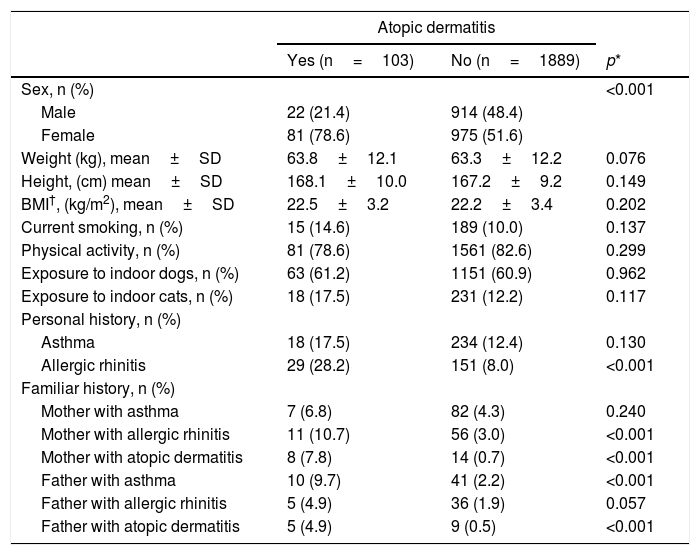
Atopic dermatitis was also more prevalent among women (Table 3). In this case, there was no significant association between atopic dermatitis and exposure to dogs or cats; furthermore, only a personal history of allergic rhinitis proved to be significantly linked to atopic dermatitis. A maternal history of rhinitis or atopic dermatitis and a paternal history of asthma and atopic dermatitis were significantly associated with the prevalence of atopic dermatitis.
Characteristics of the population with atopic dermatitis.
| Atopic dermatitis | |||
|---|---|---|---|
| Yes (n=103) | No (n=1889) | p* | |
| Sex, n (%) | <0.001 | ||
| Male | 22 (21.4) | 914 (48.4) | |
| Female | 81 (78.6) | 975 (51.6) | |
| Weight (kg), mean±SD | 63.8±12.1 | 63.3±12.2 | 0.076 |
| Height, (cm) mean±SD | 168.1±10.0 | 167.2±9.2 | 0.149 |
| BMI†, (kg/m2), mean±SD | 22.5±3.2 | 22.2±3.4 | 0.202 |
| Current smoking, n (%) | 15 (14.6) | 189 (10.0) | 0.137 |
| Physical activity, n (%) | 81 (78.6) | 1561 (82.6) | 0.299 |
| Exposure to indoor dogs, n (%) | 63 (61.2) | 1151 (60.9) | 0.962 |
| Exposure to indoor cats, n (%) | 18 (17.5) | 231 (12.2) | 0.117 |
| Personal history, n (%) | |||
| Asthma | 18 (17.5) | 234 (12.4) | 0.130 |
| Allergic rhinitis | 29 (28.2) | 151 (8.0) | <0.001 |
| Familiar history, n (%) | |||
| Mother with asthma | 7 (6.8) | 82 (4.3) | 0.240 |
| Mother with allergic rhinitis | 11 (10.7) | 56 (3.0) | <0.001 |
| Mother with atopic dermatitis | 8 (7.8) | 14 (0.7) | <0.001 |
| Father with asthma | 10 (9.7) | 41 (2.2) | <0.001 |
| Father with allergic rhinitis | 5 (4.9) | 36 (1.9) | 0.057 |
| Father with atopic dermatitis | 5 (4.9) | 9 (0.5) | <0.001 |
SD: Standard deviation.
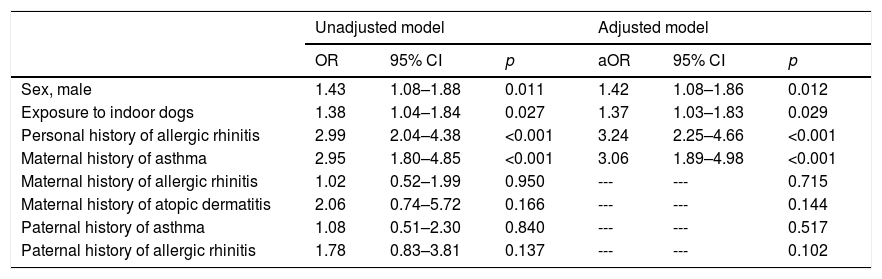
Through multivariate analyses, it was evident that exposure to indoor dogs was significantly associated with asthma prevalence (aOR=1.37; 95% CI: 1.03–1.83) (Table 4), as was the male sex (aOR=1.42; 95% CI: 1.08–1.86), a personal history of allergic rhinitis (aOR=3.24; 95% CI: 2.25–4.66), and a maternal history of asthma (aOR=3.06; 95% CI: 1.89–4.98). The population attributable risk for exposure to indoor dogs was 18%.
Multivariate analysis between exposure to outdoor dog (independent variable) and the prevalence of asthma (dependent variable) using binary logistic regression.
| Unadjusted model | Adjusted model | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | aOR | 95% CI | p | |
| Sex, male | 1.43 | 1.08–1.88 | 0.011 | 1.42 | 1.08–1.86 | 0.012 |
| Exposure to indoor dogs | 1.38 | 1.04–1.84 | 0.027 | 1.37 | 1.03–1.83 | 0.029 |
| Personal history of allergic rhinitis | 2.99 | 2.04–4.38 | <0.001 | 3.24 | 2.25–4.66 | <0.001 |
| Maternal history of asthma | 2.95 | 1.80–4.85 | <0.001 | 3.06 | 1.89–4.98 | <0.001 |
| Maternal history of allergic rhinitis | 1.02 | 0.52–1.99 | 0.950 | --- | --- | 0.715 |
| Maternal history of atopic dermatitis | 2.06 | 0.74–5.72 | 0.166 | --- | --- | 0.144 |
| Paternal history of asthma | 1.08 | 0.51–2.30 | 0.840 | --- | --- | 0.517 |
| Paternal history of allergic rhinitis | 1.78 | 0.83–3.81 | 0.137 | --- | --- | 0.102 |
OR: odds ratio obtained by logistic regression. aOR: adjusted odds ratio. CI: Confidence interval.
Unadjusted model: method “Enter”. Adjusted model: method “Forward conditional” (If p> 0.05 then variables were excluded from model).
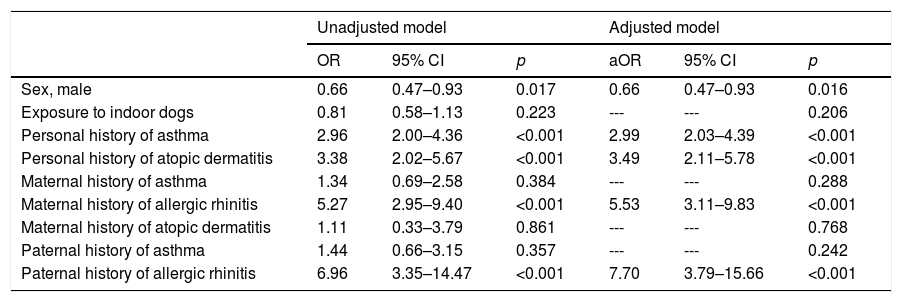
In Table 5, we show the factors that are associated with the prevalence of allergic rhinitis. In contrast to the results for asthma, there was no association between exposure to indoor dogs and allergic rhinitis. However, other associated factors included male sex (ORa=0.66; 95% CI: 0.47–0.93), a personal history of asthma (aOR=2.99; 95% CI: 2.03–4.39) and atopic dermatitis (aOR=3.49; 95% CI: 2.11–5.78), as well as a maternal or paternal history of allergic rhinitis (aOR=5.53; 95% CI: 3.11–9.83 and aOR=7.70; 95% CI: 3.79–15.66, respectively).
Multivariate analysis between exposure to outdoor dog (independent variable) and the prevalence of allergic rhinitis (dependent variable) using binary logistic regression.
| Unadjusted model | Adjusted model | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | aOR | 95% CI | p | |
| Sex, male | 0.66 | 0.47–0.93 | 0.017 | 0.66 | 0.47–0.93 | 0.016 |
| Exposure to indoor dogs | 0.81 | 0.58–1.13 | 0.223 | --- | --- | 0.206 |
| Personal history of asthma | 2.96 | 2.00–4.36 | <0.001 | 2.99 | 2.03–4.39 | <0.001 |
| Personal history of atopic dermatitis | 3.38 | 2.02–5.67 | <0.001 | 3.49 | 2.11–5.78 | <0.001 |
| Maternal history of asthma | 1.34 | 0.69–2.58 | 0.384 | --- | --- | 0.288 |
| Maternal history of allergic rhinitis | 5.27 | 2.95–9.40 | <0.001 | 5.53 | 3.11–9.83 | <0.001 |
| Maternal history of atopic dermatitis | 1.11 | 0.33–3.79 | 0.861 | --- | --- | 0.768 |
| Paternal history of asthma | 1.44 | 0.66–3.15 | 0.357 | --- | --- | 0.242 |
| Paternal history of allergic rhinitis | 6.96 | 3.35–14.47 | <0.001 | 7.70 | 3.79–15.66 | <0.001 |
OR: odds ratio obtained by logistic regression. aOR: adjusted odds ratio. CI: Confidence interval.
Unadjusted model: method “Enter”. Adjusted model: method “Forward conditional” (If p > 0.05 then variables were excluded from model).
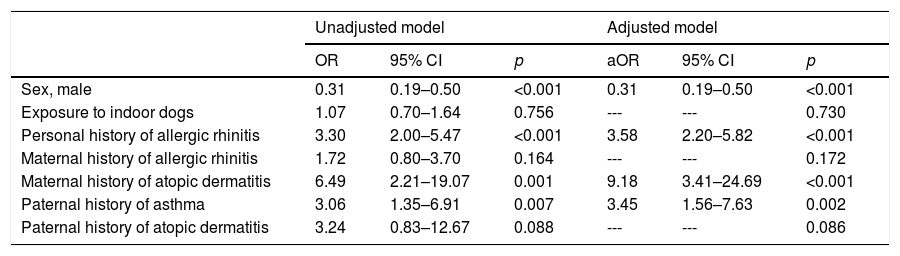
Lastly, there was no association between exposure to indoor dogs and atopic dermatitis. The factors that were found to be associated with atopic dermatitis were male sex (ORa=0.31; 95% CI: 0.19–0.50), allergic rhinitis (aOR=3.58; 95% CI: 2.20–5.82), a mother with atopic dermatitis (aOR=9.18; 95% CI: 3.41–24.69), and a father with asthma (aOR=3.45; 95% CI: 1.56–7.63) (Table 6).
Multivariate analysis between exposure to outdoor dog (independent variable) and the prevalence of atopic dermatitis (dependent variable) using binary logistic regression.
| Unadjusted model | Adjusted model | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | aOR | 95% CI | p | |
| Sex, male | 0.31 | 0.19–0.50 | <0.001 | 0.31 | 0.19–0.50 | <0.001 |
| Exposure to indoor dogs | 1.07 | 0.70–1.64 | 0.756 | --- | --- | 0.730 |
| Personal history of allergic rhinitis | 3.30 | 2.00–5.47 | <0.001 | 3.58 | 2.20–5.82 | <0.001 |
| Maternal history of allergic rhinitis | 1.72 | 0.80–3.70 | 0.164 | --- | --- | 0.172 |
| Maternal history of atopic dermatitis | 6.49 | 2.21–19.07 | 0.001 | 9.18 | 3.41–24.69 | <0.001 |
| Paternal history of asthma | 3.06 | 1.35–6.91 | 0.007 | 3.45 | 1.56–7.63 | 0.002 |
| Paternal history of atopic dermatitis | 3.24 | 0.83–12.67 | 0.088 | --- | --- | 0.086 |
OR: odds ratio obtained by logistic regression. aOR: adjusted odds ratio. CI: Confidence interval.
Unadjusted model: method “Enter”. Adjusted model: method “Forward conditional” (If p > 0.05 then variables were excluded from model).
In this study, we show that in addition to male sex, a personal history of allergic rhinitis and maternal asthma, exposure to indoor dogs during the previous year is associated with the prevalence of asthma in late adolescents. Neither allergic rhinitis nor atopic dermatitis were found to be associated with exposure to dogs or cats. Moreover, we observed that a personal history of asthma or atopic dermatitis and allergic rhinitis in one of the parents increased the risk for allergic rhinitis, while male sex decreased the risk. Similarly, we found that a personal history of allergic rhinitis, maternal atopic dermatitis and paternal asthma are risk factors for atopic dermatitis, but male sex seems to be a protective factor. Lastly, we observed a negative association between male sex and atopic dermatitis; however, a positive association between a personal history of allergic rhinitis, maternal atopic dermatitis and paternal asthma was found.
Overall, living with pets has different effects on the health and well-being of people; for example, pets have been used as a source of motivational or physical therapy, as protective agents against some diseases, including cardiovascular diseases, or as agents that reduce psychological changes. Therefore, pets are important actors that facilitate interpersonal relationships.1 However, despite all of the benefits pets offer, in this study, we found that exposure to indoor dogs during the year that preceded our survey was an independent factor associated with asthma prevalence. Although the published evidence is still contradictory, it is interesting that the exposure of an individual to pets seems to be a relevant aspect in the development of allergic diseases. For example, exposure to dogs and cats at birth has not been shown to increase the risk of allergic diseases.10 In contrast, it has been proven that the same early exposure is a risk factor for asthma, rhinoconjunctivitis and atopic dermatitis.3–5 However, when exposure to dogs and cats occurs at an early stage of life, current or not, it does not increase the risk of asthma among children.11 Recently, it was observed that children who were exposed to farm animals during the first year of life had a reduced risk of asthma when they were six years of age.12 In our population, it has been shown that when children have early exposure to dogs (during the first year of life), they are less likely to develop atopic dermatitis, although no relationship was found with asthma or allergic rhinitis.13 These same findings have been previously observed in children from Denmark.14 The main question is: What were the factors that led to the exposure to dogs to become an independent variable associated with asthma among the adolescents in our study? The answer might be found in the primordial elements, the first of which is whether the adolescent was already sensitized to dog fur, with or without having developed allergic rhinitis, as atopy is an agent that has been previously linked to the development of asthma.15,16 A second explanation is that exposure to bacterial endotoxins may increase the risk of exercise-induced bronchospasms but decrease the risk of atopy in subjects with asthma.17–19 In the light of the available evidence, it appears that introducing pets into a child’s life at an early age as a strategy to reduce the risk of allergic diseases is not very effective. It would seem to be more beneficial to introduce a pet during the first few months of life, or even during the prenatal stage.
Additionally, genetic inheritance and sex emerged as independent factors associated with asthma prevalence among adolescents. First, a genetic predisposition is the most consistent factor that has been linked to allergic diseases. However, hereditary patterns are complex and do not appear to behave similarly throughout the different regions of the world. In our study, a maternal history of asthma made the development of asthma three-fold more likely during adolescence, a finding that is consistent with previous studies.6,20 Second, we observed that gender plays a role in the prevalence of allergic diseases; however, sex does not behave in a consistent manner; males manifested a higher frequency of asthma, but females were more frequently affected by allergic rhinitis and atopic dermatitis. It is generally accepted that during childhood, allergic diseases are more common among boys; however, with the onset of puberty, they become more prevalent in women, a pattern that is maintained throughout adulthood; in Europe, several cohort studies have proposed this hypothesis. Notably, the effect of an individual’s sex is more pronounced when the adolescent simultaneously has asthma and allergic rhinitis.21
In order to interpret our results, we recommend taking the following limitations into careful consideration: 1) We utilized the responses from a questionnaire as an instrument to establish a diagnosis of allergic diseases, and we worked under the assumption that these allergic diseases had been previously diagnosed by a physician; similarly, it should be mentioned that the ISAAC questionnaire was validated for children aged 13 and 14 years. 2) We did not include an in-depth inquiry about exposure to dogs, that is to say, we did not ask how long each subject had been exposed to an indoor dog; nor did we ask about the number of dogs within each household. Furthermore, we did not establish an aeroallergen concentration or the endotoxins that came from these pets. 3) Exposure to dogs and cats may have triggered an allergic sensitization, although this was not confirmed through an IgE serum count. 4) When we inquired about exposure to dogs during the year that preceded our study, we assumed that these households were in the habit of keeping their pets indoors; yet we did not verify this matter, which means that we cannot be certain whether asthma or other allergic diseases were triggered prior to the subject’s exposure to pets. 5) Although the sample size was not justified a priori, a statistically significant association was found between dog exposure and asthma prevalence, indicating that statistical power was sufficient for these variables. However, this was not the case for allergic rhinitis or atopic dermatitis. It should be noted that the lack of association with respect to these two variables could also have been due to an insufficient sample size; therefore, new studies with an explicit sample size are recommended. 6) Attributable risk should be interpreted with caution since it was calculated in a cross-sectional study rather than a case-control study. However, due to the random sample, we can assume that subjects with asthma are representative of the population.
Our results suggest that exposure to indoor dogs during the year that preceded our survey was significantly associated with the prevalence of asthma in adolescents aged 15–18 years; our results also indicate that the proportion of asthma cases attributable to exposure to dogs is low (18%). We also found additional associated factors, such as sex, family history of atopy, and personal atopy. In relation to exposure to cats, we did not observe any direct link between these pets and asthma, allergic rhinitis, or atopic dermatitis. Further studies aimed at determining the extent of exposure to dogs and cats and its contribution to the development of allergic diseases, as well as the moment at which exposure begins, may be able to shed further light on the role that interacting with these household pets plays in the development of allergic diseases.
Conflicts of interestThe authors have no conflict of interest to declare.
Funding sourceNone.