Helminth infections and allergies are diseases with intense Th2 lymphocytes participation and characterised by a high IgE and Interleukin-(IL) IL-4, IL-5 production and eosinophilia. However, helminths also induce IL-10 production, which may alter the outcome of allergic diseases in infected patients.
ObjectiveThis experimental study analyses the relationship between IL-10 production by cell culture from geohelminth infected and non-infected children and specific IgE to Ascaris lumbricoides (Asc) or Blomia tropicalis (BT).
MethodsIL-10 content in supernatant from peripheral blood mononuclear cell culture from nine helminth infected and eleven non-infected patients was determined by ELISA after in vitro stimulation with Asc or BT extracts.
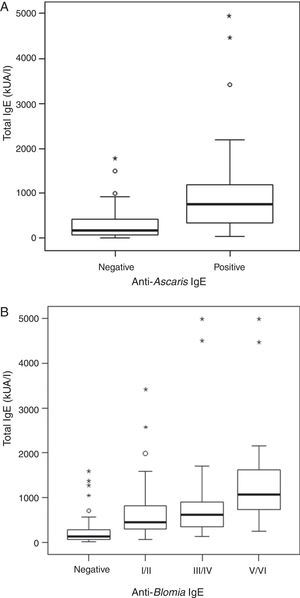
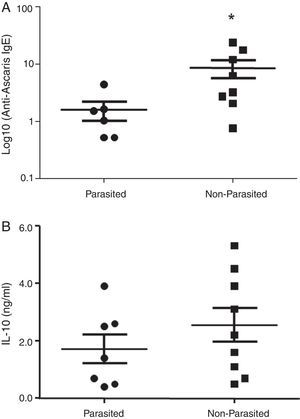
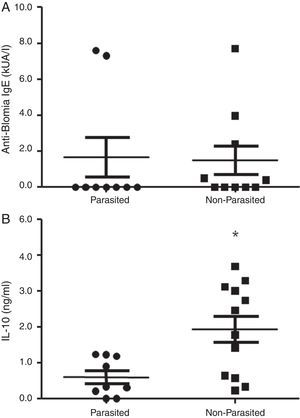
ResultsA positive association was observed between total IgE levels and anti-Ascaris and anti-Blomia tropicalis specific IgE, independent of infection status. For both helminth-infected and non-infected groups, there was no difference in IL-10 production in response to Asc extract, even though anti-Ascaris IgE levels were higher in the latter group. In response to BT stimulus, a lower production of IL-10 by the geohelminth-infected group was observed, but with no relationship between IL-10 production and specific IgE to BT.
ConclusionThe results suggest that anti-Ascaris IgE in non-infected patients may be associated to a resistance to parasites. Levels of specific IgE to parasite antigens or B. tropicalis allergen were not impaired by IL-10 production in children from an urban area in which geohelminthiasis is endemic.
Allergic diseases are mediated by type Th2 immune response (IL-4, IL-5, IL-13, eosinophilia and high IgE levels) as a consequence of the complex interaction between genetic predisposition and the constant contact with environmental allergic proteins.1–3 Proteins derived from helminths, e.g. Ascaris lumbricoides, also share the property of stimulating a Th2 response in the host, accompanied by a significant IgE production. During helminth infection, a large quantity of IgE is produced against parasitic antigens that together with a polyclonal stimulation leads to an increase in total IgE levels, including anti-allergens IgE.4 Similarly, it has been observed that atopic patients are more likely to produce IgE against helminth proteins and present a degree of resistance, especially to a high parasitic load.5 Moreover, it has also been demonstrated that allergic and parasite proteins share many analogies.6
Although they share immunological reactions such as total IgE hyperproduction, helminthiasis and allergic diseases differ in their capacity to modulate the immune response of the host. Throughout the acute phase of helminth infection there is an IgE-mediated inflammation and symptoms similar to those of allergy (urticarial reactions, bronchospasms caused by larval migration and eosinophil tissue recruitment) may also happen.2 After a period of parasitic aggression there is a host adaptation. During this chronic phase, parasites may stimulate regulatory T cells with a significant production of IL-10 which contribute to the suppression of the Th2 response against parasites7 and may also alter the response to environmental allergens.
Helminth immunomodulation has been the basis of several studies that showed an inverse relationship between parasites and allergies.8 These studies indicate an increase in allergic diseases in urban centres, where the prevalence of parasitic infections are very low, in contrast to rural or urban areas with poor sanitation conditions, where parasitic diseases are highly prevalent.9Focusing on allergic diseases in patients with intestinal parasites, studies have demonstrated an increase in total IgE levels, accompanied by the presence of anti-Ascaris IgE, and an inverse relationship between total IgE and positive cutaneous hypersensitivity test in patients with high parasitic loads.10,11 According to several authors, rather than being considered as a response marker to geohelminth and parasitic symptomatology, anti-Ascaris IgE has been regarded as a risk factor for atopic disease and as a possible marker for or propensity to allergic disease.12–14
The immunomodulatory role of IL-10 in the IgE-dependent immune response has been demonstrated, not only in the induction phase (B lymphocytes IgE production),15 but also in the effector phase (mast cell and eosinophil activation).16 However, very few studies have examined the role of this cytokine during geohelminth infections and its relationship with allergies and IgE levels.17,18 Therefore, the aim of this experimental study was to evaluate the IL-10 production capacity of peripheral blood mononuclear cells from allergic parasitised and non-parasitised children living in an urban area of Northeast Brazil and to evaluate the relationship between parasitic infection, total and specific anti-Ascaris and anti-Blomia IgE levels.
Materials and methodsStudy design and populationFrom a clinical study involving 123 children, a convenience sample of 20 children (9 parasited and 11 non-parasited by geohelminths) was selected for an exploratory study of cellular culture to evaluate IL-10 production. All included children had respiratory allergic manifestations (rhinitis and/or asthma) and a positive prick test to B. tropicalis or D. pteronyssinus antigens.
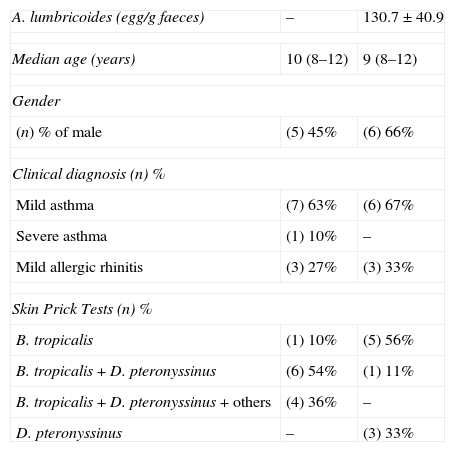
Children's age varied between 9 and 12 years (median 10 years) and there were nine females. For the nine parasitised individuals, in which only A. lumbricoides were found in stools were with a low parasitic load (<500 eggs/gram faeces). No parasites were found in three stool samples of 11 children (non-parasitised group) (Table 1).
Demographic data from asthmatic individuals infected with Ascaris lumbricoides or non-infected.
| A. lumbricoides (egg/g faeces) | – | 130.7±40.9 |
| Median age (years) | 10 (8–12) | 9 (8–12) |
| Gender | ||
| (n) % of male | (5) 45% | (6) 66% |
| Clinical diagnosis (n) % | ||
| Mild asthma | (7) 63% | (6) 67% |
| Severe asthma | (1) 10% | – |
| Mild allergic rhinitis | (3) 27% | (3) 33% |
| Skin Prick Tests (n) % | ||
| B. tropicalis | (1) 10% | (5) 56% |
| B. tropicalis+D. pteronyssinus | (6) 54% | (1) 11% |
| B. tropicalis+D. pteronyssinus+others | (4) 36% | – |
| D. pteronyssinus | – | (3) 33% |
IL-10 content in supernatant from peripheral blood mononuclear cell culture from patients after in vitro stimulation with B. tropicalis and Ascaris extract was determined by ELISA. Each patient had samples cultured in duplicate and for ELISA, each supernatant obtained was also measured in duplicate.
In order to assess the humoral allergic responses, total IgE, B. tropicalis-specific IgE (the most important aeroallergen in clinical study) and anti-Ascaris IgE plasma levels were measured.
Each child had three quantitative serial sample stool exams and a skin test for house dust mites (B. tropicalis and Dermatophagoides pteronyssinus). All children included in this research showed concordance between skin prick-test and specific IgE to B. tropicalis.
The study was approved by the Health Sciences Research Ethics Committee at UFPE (CEP/CCS/UFPE). Those responsible for the children signed a written informed consent to participate in the project.
Parasitological stool examinationHelminth eggs were examined using the spontaneous sedimentation method (Hofmann) in three stool samples preserved in formalin 10% on alternate days. The Kato-Katz method was used to establish the number of eggs per gram of faeces (OPG).
In vitro IL-10 stimulation and measurementVenous blood samples were collected from the cubital vein in heparinised tubes and mononuclear cells from the peripheral blood were separated by density gradient centrifugation – Ficoll-Hypaque (Sigma). The cell suspensions were adjusted to a final concentration of 3×106 cells/mL in RPMI 1640 medium containing 10% foetal calf bovine, 100U/mL penicillin, 100mg/mL streptomycin, 2mmol/L l-glutamine and 30mmol/L HEPES (all from Sigma–Aldrich). The cells were distributed in 48-well tissue culture plates (Costar) in duplicate and stimulated with Ascaris sp. (250(g/mL) or B. Tropicalis (25(g/mL) in a humidified CO2 (5%) incubator for 72h. After incubation, the supernatants were collected and maintained at −20°C for later measurement of IL-10 cytokine. For cell stimulation, a lyophilised Ascaris adult worms extract prepared as previously described19 re-suspended in phosphate-buffered saline (PBS) at the moment of use and a commercial B. tropicalis antigen (ALC Farmacêutica, São Paulo, Brazil) were used.
Cell culture supernatant IL-10 was measured by sandwich ELISA technique with the following monoclonal antibodies: anti-IL-10 capture JES3-12G8 and biotinylated JES3-19F1. Binding of biotinylated antibodies was detected using streptavidin-peroxidase conjugate and chromogen ABTS (-2,2 azinobis (3-ethylbenzthiazoline-6 sulfonic acid) Sigma) plus H2O2 (Sigma). The plates were read (410nm) in an automated ELISA reader. Samples were quantified by comparison with a standard curve of purified recombinant cytokines. The limit of detection for IL-10 was 0.31ng/mL. There was not spontaneous production of this cytokine in the cultive only with medium.
Detection of IgE antibodiesFor the detection of IgE antibodies, the plasma of each patient was submitted to automated enzyme-linked fluorescent assay by the CAP System (PHADIA Diagnostics). The levels of specific IgE to B. tropicalis were expressed in five classes according to the standards established by Phadia, with a lower limit of detection of 0.35IU/ml.
Statistical analysisThe Kruskal–Wallis test for multivariate analysis and Mann–Whitney test for dichotomous variables were adopted for comparative analysis and a 5% alpha error was assumed.
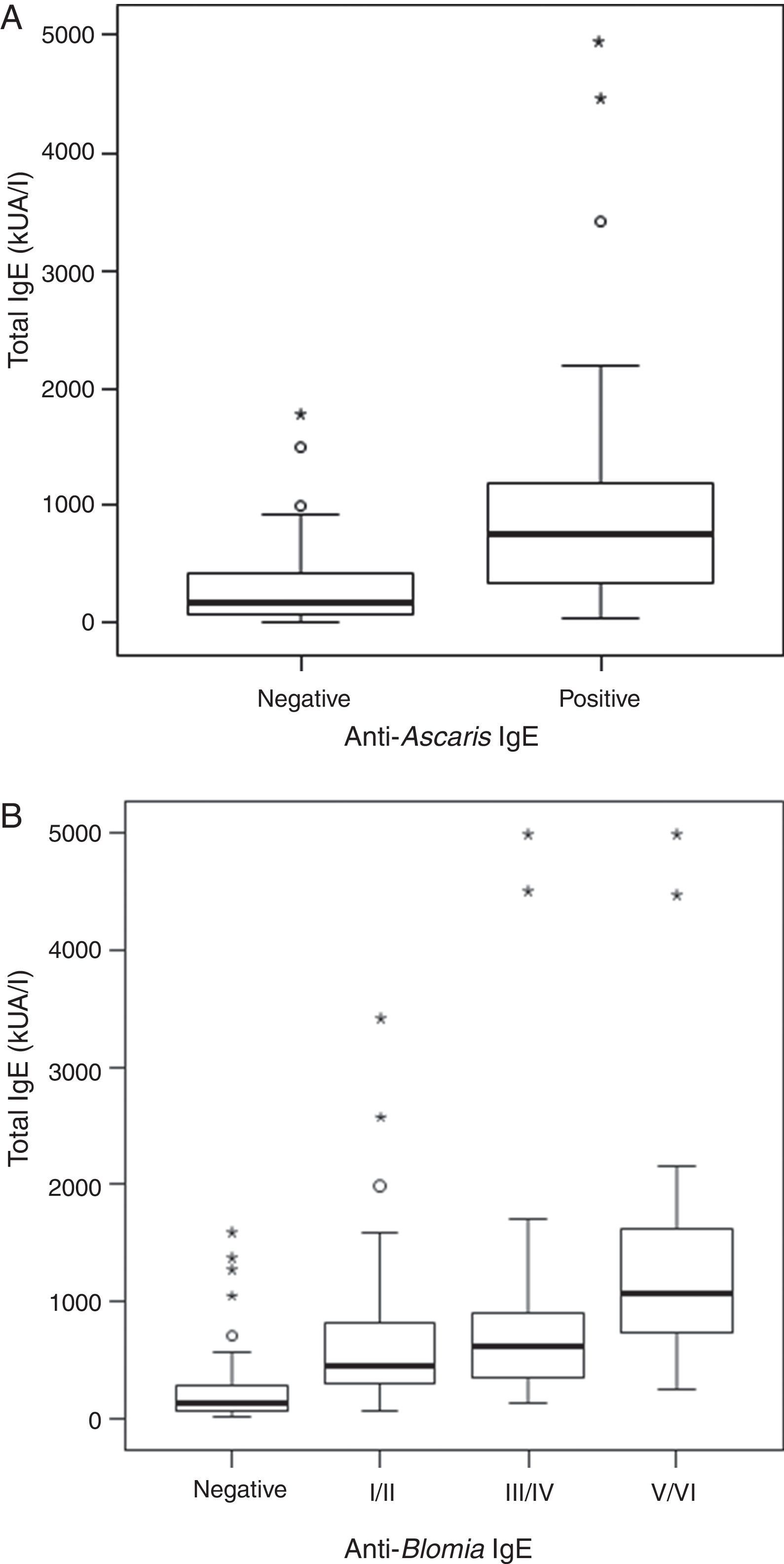
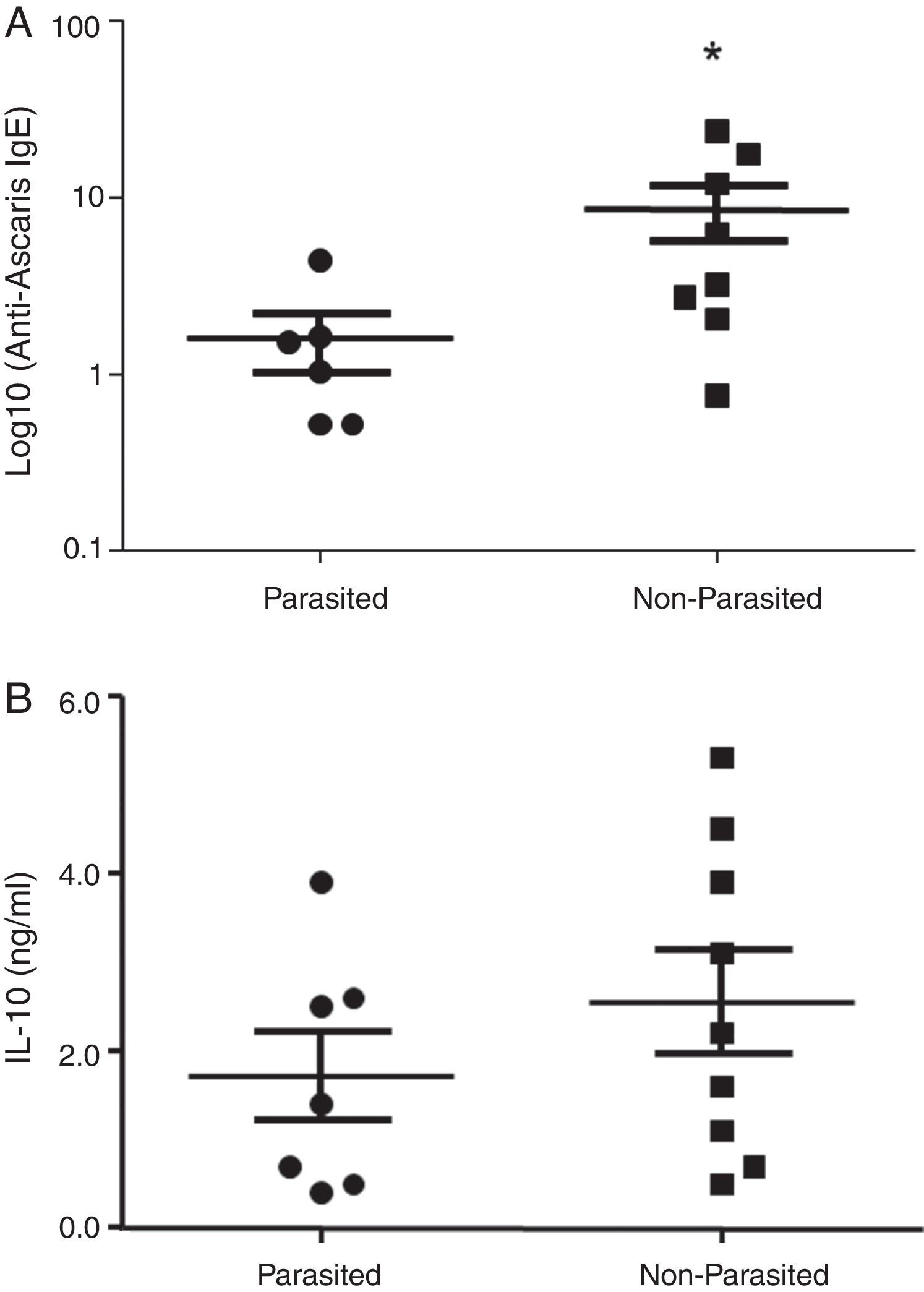
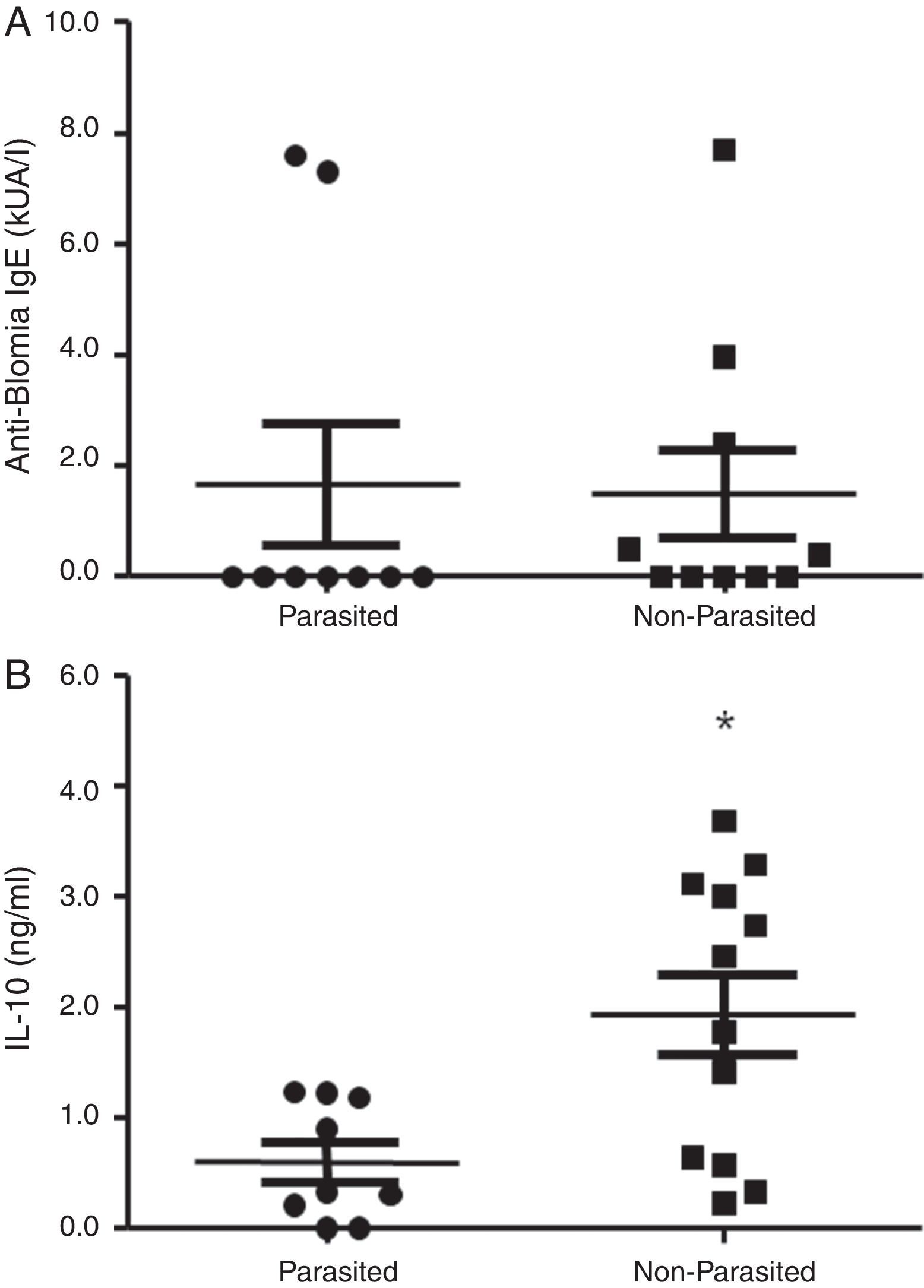
ResultsThe serological analysis showed a positive association between total IgE and anti-Asc IgE (Fig. 1A) and anti-BT IgE levels (Fig. 1B). It was observed that anti-Ascaris IgE levels in non-parasited subjects were significantly higher than in the parasited patients (Fig. 2A), however, there was no difference in IL-10 synthesis in response to Ascaris antigen between groups (Fig. 2B). There was no statistical difference in specific B. tropicalis IgE levels between parasited and non-parasited patients (Fig. 3A). However, there was a significantly higher production of IL-10 by peripheral mononuclear cells of non-infected compared to infected patients when stimulated with B. tropicalis antigen (Fig. 3B).
Despite different aetiologies, both allergic and helminthic diseases have IgE as the main molecule of the effector immune response consequent to Th2 cytokines stimulation. In this experimental study, nine geohelminth-infected and 11 non-infected atopic patients with clinical manifestations of asthma and/or rhinitis were evaluated for total and specific IgE for A. lumbricoides and B. tropicalis. It was demonstrated that in patients infected with A. lumbricoides or even those currently free of this infection, but living in an urban environment with low socioeconomic status and poor sanitation conditions, total IgE levels were high and were accompanied by high levels of specific IgE to A. lumbricoides and/or B. tropicalis. Infected subjects demonstrated lower levels of anti-Ascaris IgE and this observation was not related to stimulated mononuclear cell IL-10 production. Likewise, this cytokine seems not to have interfered with the sensitivity to the principal allergen of B. tropicalis.
Given the complex relation between helminthiasis and allergic diseases, different investigations have used total and specific IgE, along with cutaneous tests, as parameters to assess the sensitivity of subjects.12–14 Moreover, IL-10 levels have been assessed in order to understand the possible role of intestinal parasites against the development of allergic diseases. Although the generation of regulatory T cells has been described in Ascaridiasis,17,18 studies related to the nematode A. lumbricoides demonstrated that IL-10 does not seem to be the main immunomodulatory pathway for this worm, neither for homologous antigens (the parasite itself)20,21 nor for heterologous antigens (such as the allergens).17,22
In the present study, patients free of infection, even after repeated anti-helminthic treatment, maintained higher levels of anti-Ascaris IgE. This fact was also observed by Linch et al. (1997) in Venezuelan subjects with low parasite loads after infections treatment.12 These last authors suggested that this condition may be due to the continuous re-stimulation by evolving forms of the worm or that particularly in persons with an atopic genetic disposition and so, in this context, the higher levels of anti-Ascaris IgE would seem to reflect not only a marker of previous infection, but also a protective factor.12,23 In fact, some studies have used the anti-Ascaris IgG isotype (not IgE) as a marker for chronic infections,11,24 whereas some protective functions of the specific IgE molecule seem to contribute to the immunity of the host against different stages of the life cycle of the parasite (alterations in intestinal mobility and mucus production, associated to Th2 cytokines responses (IL-4, IL-13) and mast cell activation.25,26
Furthermore, an analysis of the isotypic profile against antigenic molecules of A. lumbricoides demonstrates that in both non-infected patients and those with a low parasitic load, there were higher levels of specific IgE, thus suggesting a protective role for this immunoglobulin isotype.27 However, it should also be considered that the genetic predisposition in atopic/allergic subjects is a determinant factor for a Th2 response and anti-helminth IgE production.5,28
Mononuclear cells of infected patients had a lower IL-10 production after stimulation with B. tropicalis allergen, but no difference was observed in the levels of specific IgE to B. tropicalis between infected and non-infected individuals. These results are in agreement with other investigations that ruling out the relationship between IgE production and IL-10 levels in A. lumbricoides infection. 17,18
B. tropicalis is the most common aeroallergen in the city where the study was conducted, and the standardised extract of this mite presented a greater positivity to the immediate cutaneous hypersensitivity test and specific IgE.29 The correlation between total IgE, anti-Ascaris IgE and anti-B. tropicalis IgE levels corroborate the fact that an immune response may be elicited to antigens shared by parasites and allergens and even have clinical consequences such as aggravating wheezing and other asthmatic symptoms in patients infected by geohelminths, especially A. lumbricoides.6
In spite of the fact that study groups were too small to provide evidence that in helminth infection and allergen sensitivity the IL-10 does not play an immunoregulatory role, it is possible to raise this hypothesis. Furthermore, it motivates the interest for other regulatory mechanisms induced by geohelminths against non-related antigens, such as allergens. In poor communities, where there is a high probability of parasitic re-infection, the observed higher levels of anti-Ascaris specific IgE in non-infected individuals may suggest a protection gain in the host-parasite relationship.
Ethical disclosuresProtection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Patients’ data protectionThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThe authors have no conflict of interest to declare.
This work was supported by Ministério da Saúde – MS, Ministério da Ciência e Tecnologia – MCT do Governo Federal Brasileiro and Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (processo no. 402666/2005-4).
The authors declare that the procedures of the study were approved by the Health Sciences Research Ethics Committee at UFPE (CEP/CCS/UFPE) and applicable local regulations; assent and written informed consent were obtained from each parent or legal guardian before study procedure was initiated. The author for correspondence is in possession of this document. The authors declare that they have followed the protocols of their work centre on the publication of patient data.