Antioxidant intake changes have been implicated with the increase in asthma and allergies outcomes, but no clear association has been revealed. In this cross sectional study, the overall effect of antioxidants on asthma and allergic diseases was studied.
MethodsData from the cohorts of the phase II ISAAC survey (2023 children 9–10 years old) in two metropolitan Greek cities were analysed. Using a semi-quantitative food frequency questionnaire, an Antioxidant Eating Index (AEI, range 0–6) was created with the pro-antioxidant (vegetables, fruits, fresh juice, fish) and the non-antioxidant (meat, burgers) food intake and was evaluated with allergic diseases. Higher values of the score suggest closer to an “antioxidant” and lesser to a “saturated fatty” diet.
ResultsPrevalence of lifetime and current asthma, current rhinitis and sensitisation were higher in Thessaloniki compared to Athens. The AEI score of the entire cohort was 4.2±1.2 (median 4.0) and was higher in Athens compared to Thessaloniki (4.3±1.2 vs. 4.0±1.2, p=0.001) and in girls than boys (4.3±1.1 vs. 4.0±1.2, p=0.001). AEI was inversely associated with lifetime asthma (OR: 0.87, 95%CI 0.77, 0.99) in either cities independently of other cofounders such as family history, sensitisation, exercise, house smoking, breast feeding, pet or dampness in houses. No association with other allergic disease or sensitisation was detected.
ConclusionAntioxidant foods seem to be a non-pharmacological, protective dietary pattern for asthma development in children irrespectively of atopy or heredity; AEI was a rough indicator and the role of antioxidants in allergic diseases is still under consideration.
Since the mid 1990s when Seaton et al.,1 hypothesised that nutrition transition to a westernised dietary pattern may be responsible for the increase in asthma prevalence, several studies have investigated the association between food intake and allergy incidence, reporting controversial results.2–8 Specific nutrients have also documented effects on immune function and risk of allergic diseases.5,9–11 The International Study of Asthma and Allergy in Children (ISAAC) was the largest study which indicated that healthy food choices, enriched in fruit, vegetables and fish, were associated with lower prevalence of current wheeze and asthma, and not with sensitisation.12 Recent meta analyses, even with weak epidemiologic evidence, supported the positive effect of vitamin A, D, and E, zinc, fruit and vegetable consumption, as well as the Mediterranean diet for the prevention of asthma.13,14 However, other review studies could not come to robust conclusions or fully appreciate the role of overall dietary habits, like antioxidants, in asthma and allergic outcomes.15–17
Both diet habits and asthma prevalence have dramatically changed in Greece during the last decades. In a worldwide study where the trends of adherence to the Mediterranean diet between 1960s and 2000s were evaluated, Greece proved to experience the greatest decrease compared to other countries.18 During the past decades, a greater than fourfold increase in asthma prevalence in Greek children was also noticed19 and nearly half of the infants with late onset asthma had persistent symptoms up to adulthood.20 Even more, different prevalence between large cities within the country has been reported.21
Based on the aforementioned considerations, a research hypothesis was stated, whether there is a relationship between childhood asthma prevalence and dietary changes; and moreover, which components of the food groups play a major role. Putting the antioxidant foods up front, an attempt was made to evaluate their total association with childhood asthma and allergies in two metropolitan Greek cities with different asthma prevalence.
Experimental methodsStudy sampleDuring October 2000–November 2001, 2023 Greek schoolchildren, 1000 from Athens, 1023 from Thessaloniki, 9–10 years of age (47.9% boys) participated in the ISAAC-II survey. In brief, children were selected from 43 primary schools in Athens (28% of the total schools) and 31 primary schools in Thessaloniki (27% of the total schools). The selection of schools was random and based on the school listings provided by the Ministry of Education. All children from each school were asked to participate. The participation rates were 85% in Athens and 63% in Thessaloniki. Geo-climatological information and asthma prevalence of Athens and Thessaloniki have been recently reported.21 Briefly, Athens being located in the centre of Greece, is a dry city with increased air pollution during summertime whereas Thessaloniki, the second largest city, located in the north of the country, experiences higher humidity and increased air pollution during winter months.
Evaluation of asthma symptoms, rhinitis and eczema, and dietary habitsAll participated children answered the ISAAC-II questionnaire, were tested with skin prick testing (SPT) to seven common aeroallergens produced by ALK (Horsholm, Dermark/mixed grass pollen, mixed tree pollen and olive tree pollen, Dermatophagoides pteronyssinus, Dermatophagoides farinae, Alternaria tenuis, cat dander) and were examined for flexural dermatitis. The questionnaire, the performed SPTs and clinical assessment are widely described in the ISAAC study.22 Lifetime asthma was defined if a specialist doctor reported cough or wheeze as asthma and current asthma was defined if asthmatic symptoms were presented in the last 12 months. Atopic asthma was reported in children with current wheeze and sensitisation to any aeroallergens. Lifetime rhinitis was reported if sneezing, running nose or nose conjunction were present in the absence of any infection and current rhinitis if these symptoms were reported in the last 12 months. Again, allergic rhinitis was defined if any sensitisation was co-found. Lifetime eczema was reported if itching rash was coming and go for more than six months and current eczema if the rash was reported in the last 12 months. Atopic was the eczema with sensitisation. Dietary habits were also evaluated through a semi-quantitative food frequency questionnaire which consisted of questions about all the major antioxidants food groups (fish, fresh fruit, cooked vegetables and fresh fruit juices) and about the consumption of red meat and burgers as indicators of the saturated fats’ diet habits through the questions ‘How often, on average, does your child consume i.e. fresh fruits? Never, less than 1/w, 1–2/w, 3–6/w, once a day’.
Antioxidant foods intake assessmentA simple, diet-specific score was developed to evaluate the level of consumption of antioxidant foods. In particular, the consumption of four major food groups (red meat, fish, raw green vegetables, fruits and fresh juices) as well as burgers was taken into account for developing the score. Following the international recommendations as endorsed by the American Academy of Pediatrics,23 consumption of foods with known antioxidant capacity (i.e., fish, raw green vegetables, fruits and juices) was scored equal to 0 if it was below the recommended and 1, if it was above. The opposite scoring system was given for red meat more than three times a week, and burger consumption more than once a week; since although meat contains selenium and zinc, studies have reported clear adverse effect on asthma outcomes.12,21,24–26 Moreover in the current univariate analysis, meat intake and products were related to asthma symptoms. Thus, an index (Antioxidant Eating Index, AEI) with a theoretical score between 0 and 6 was developed; higher values of the score suggest closer to an “antioxidant” and lesser to a “saturated fatty” diet. Because of the lack of detailed information in consumption, the authors used the proposed score only as a total rough indicator of an antioxidant diet and not as a tool to measure the exact level of antioxidants consumed.
BioethicsThis project was laid down according to the guidelines in the Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects and was approved by the National Institution of Bioethics committees. Children's parents or guardians were informed about the aims and design of the study and provided their consent.
Statistical analysisPrevalence of asthma and allergic diseases was defined as the ratio of cases divided by the number of participating children. Comparisons between children's asthma and allergic diseases prevalence and categorical characteristics were performed using the Pearson's chi-square test. The association between the antioxidants score and asthma symptoms and allergies was tested using nested multiple logistic regression models; three models adjusting factors such as city of origin and exercise level (model 1), parental allergy history and breast feeding (model 2) and other variables that showed significance in the univariate analyses (i.e., parental academic education, environmental indoor and outdoor factors), were estimated. The results are presented as odds ratios (OR) and their corresponding 95% confidence intervals. Hosmer–Lemeshow statistic was calculated in order to assess a model's goodness of fit. Collinearity between factors entered in each model was evaluated using the correlation coefficients of the estimates. SPSS 18.0 statistical programme (SPSS Hellas, Athens, Greece) was used to analyse the data.
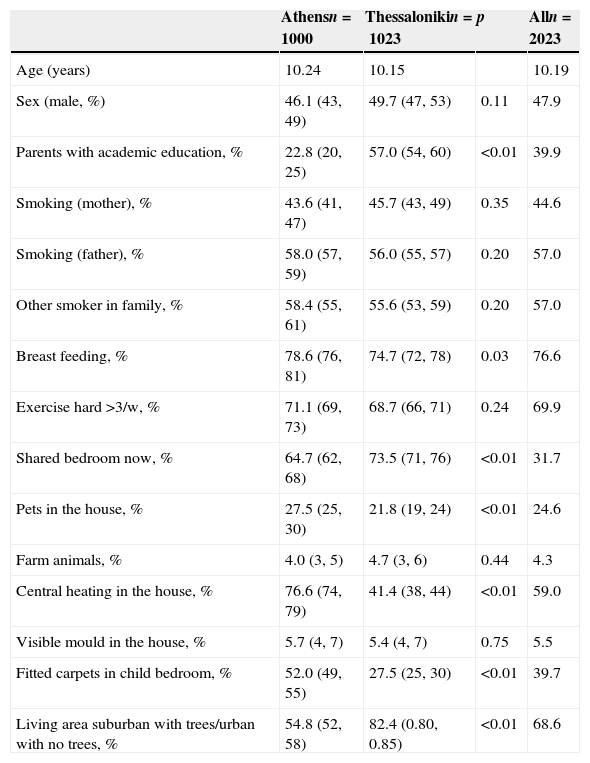
ResultsSocial and environmental characteristics of the studied sampleCharacteristics of the children and their families are presented in Table 1. In Thessaloniki, there were more parents with academic education as compared with Athens, more children shared a bedroom with siblings and were living in urban areas or suburban areas. In Athens, breast feeding was significantly higher, and more houses had central heating and covered with fitted carpets than in Thessaloniki. Pet ownership was also more frequent in Athens. No other significant differences were observed in social and environmental characteristics of the participating children.
Social and environmental characteristics of the 9–10-year-old children that participated in the study.
| Athensn=1000 | Thessalonikin=1023 | p | Alln=2023 | |
|---|---|---|---|---|
| Age (years) | 10.24 | 10.15 | 10.19 | |
| Sex (male, %) | 46.1 (43, 49) | 49.7 (47, 53) | 0.11 | 47.9 |
| Parents with academic education, % | 22.8 (20, 25) | 57.0 (54, 60) | <0.01 | 39.9 |
| Smoking (mother), % | 43.6 (41, 47) | 45.7 (43, 49) | 0.35 | 44.6 |
| Smoking (father), % | 58.0 (57, 59) | 56.0 (55, 57) | 0.20 | 57.0 |
| Other smoker in family, % | 58.4 (55, 61) | 55.6 (53, 59) | 0.20 | 57.0 |
| Breast feeding, % | 78.6 (76, 81) | 74.7 (72, 78) | 0.03 | 76.6 |
| Exercise hard >3/w, % | 71.1 (69, 73) | 68.7 (66, 71) | 0.24 | 69.9 |
| Shared bedroom now, % | 64.7 (62, 68) | 73.5 (71, 76) | <0.01 | 31.7 |
| Pets in the house, % | 27.5 (25, 30) | 21.8 (19, 24) | <0.01 | 24.6 |
| Farm animals, % | 4.0 (3, 5) | 4.7 (3, 6) | 0.44 | 4.3 |
| Central heating in the house, % | 76.6 (74, 79) | 41.4 (38, 44) | <0.01 | 59.0 |
| Visible mould in the house, % | 5.7 (4, 7) | 5.4 (4, 7) | 0.75 | 5.5 |
| Fitted carpets in child bedroom, % | 52.0 (49, 55) | 27.5 (25, 30) | <0.01 | 39.7 |
| Living area suburban with trees/urban with no trees, % | 54.8 (52, 58) | 82.4 (0.80, 0.85) | <0.01 | 68.6 |
p-Values derived from Pearson's chi square test.
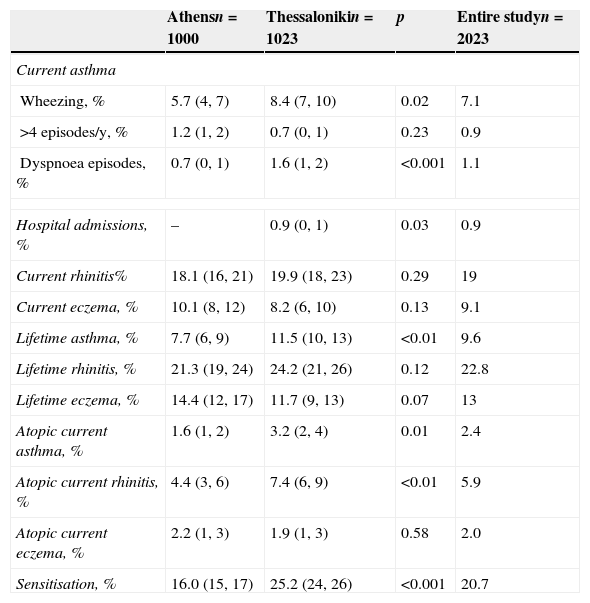
Prevalence of lifetime asthma, current asthma (atopic and non-atopic), atopic current rhinitis, severity of the disease and sensitisation were higher in Thessaloniki as compared to Athens (Table 2). There were no differences regarding lifetime or current rhinitis and any type of eczema between the two cities.
Prevalence (95% confidence interval) of asthma symptoms in 9–10-year-old children that participated in the Greek cohorts of the phase II ISAAC survey.
| Athensn=1000 | Thessalonikin=1023 | p | Entire studyn=2023 | |
|---|---|---|---|---|
| Current asthma | ||||
| Wheezing, % | 5.7 (4, 7) | 8.4 (7, 10) | 0.02 | 7.1 |
| >4 episodes/y, % | 1.2 (1, 2) | 0.7 (0, 1) | 0.23 | 0.9 |
| Dyspnoea episodes, % | 0.7 (0, 1) | 1.6 (1, 2) | <0.001 | 1.1 |
| Hospital admissions, % | – | 0.9 (0, 1) | 0.03 | 0.9 |
| Current rhinitis% | 18.1 (16, 21) | 19.9 (18, 23) | 0.29 | 19 |
| Current eczema, % | 10.1 (8, 12) | 8.2 (6, 10) | 0.13 | 9.1 |
| Lifetime asthma, % | 7.7 (6, 9) | 11.5 (10, 13) | <0.01 | 9.6 |
| Lifetime rhinitis, % | 21.3 (19, 24) | 24.2 (21, 26) | 0.12 | 22.8 |
| Lifetime eczema, % | 14.4 (12, 17) | 11.7 (9, 13) | 0.07 | 13 |
| Atopic current asthma, % | 1.6 (1, 2) | 3.2 (2, 4) | 0.01 | 2.4 |
| Atopic current rhinitis, % | 4.4 (3, 6) | 7.4 (6, 9) | <0.01 | 5.9 |
| Atopic current eczema, % | 2.2 (1, 3) | 1.9 (1, 3) | 0.58 | 2.0 |
| Sensitisation, % | 16.0 (15, 17) | 25.2 (24, 26) | <0.001 | 20.7 |
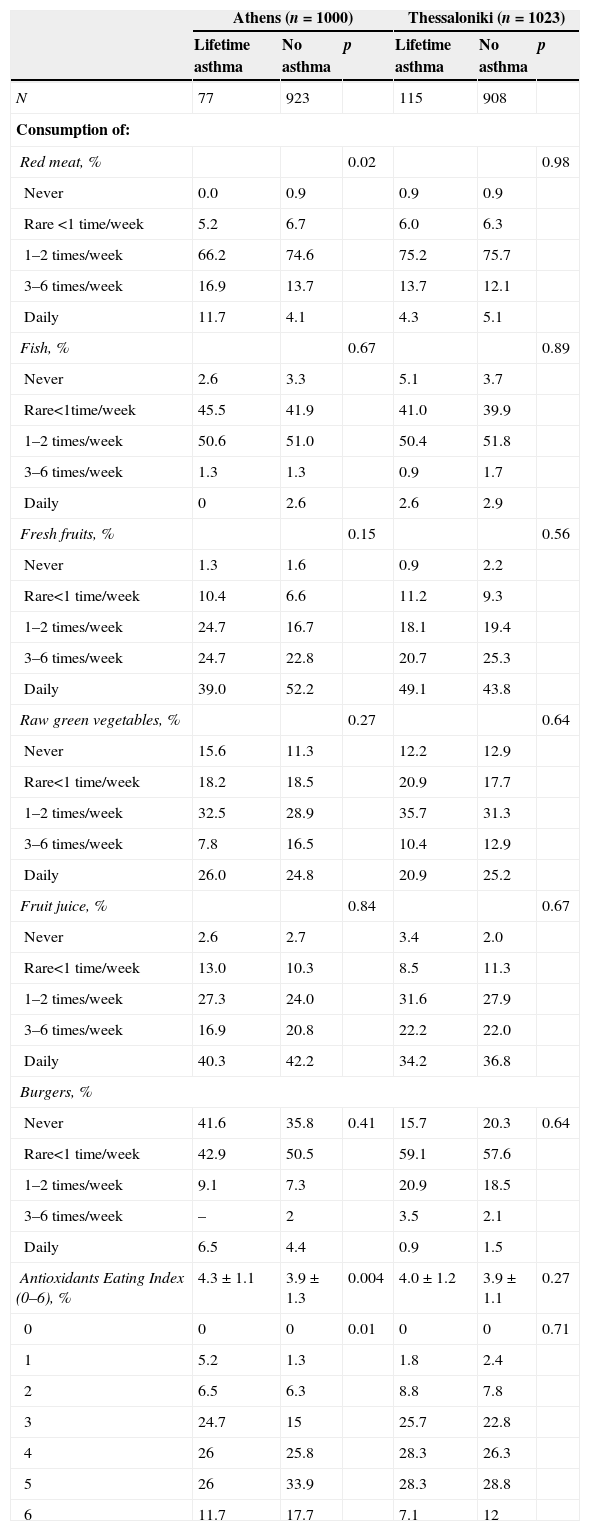
The AEI score in Greek cities was 4.2±1.2 (median 4.0). It was higher in Athens compared to Thessaloniki (4.3±1.2 vs. 4.0±1.2, p=0.001) and in girls than boys (4.3±1.1 vs. 4.0±1.2, p=0.001). Smoking was also related to AEI. Children in a smoke-free environment had higher AEI score compared to those with passive smoking (4.3±1.1 vs. 4.1±1.2, p=0.001). AEI score was not associated with exercise, parental academic education or nationality. Further detailed dietary analysis evaluated AEI and each one food component, such as meat, fish, fresh fruits, vegetables, fruit juice and burger consumption with lifetime asthma in each city (Table 3). Only red meat products consumption was associated with asthma symptoms in Athens (p=0.02). Similarly, AEI score was higher in children without asthma symptoms as compared to children with asthma in Athens (4.3±1.1 vs. 3.9±1.3, p=0.004). Other allergic diseases and sensitisation were not associated with each food components or AEI.
Weekly eating habits (times per week) and asthma symptoms prevalence in the two major cities in Greece, Athens and Thessaloniki.
| Athens (n=1000) | Thessaloniki (n=1023) | |||||
|---|---|---|---|---|---|---|
| Lifetime asthma | No asthma | p | Lifetime asthma | No asthma | p | |
| N | 77 | 923 | 115 | 908 | ||
| Consumption of: | ||||||
| Red meat, % | 0.02 | 0.98 | ||||
| Never | 0.0 | 0.9 | 0.9 | 0.9 | ||
| Rare <1 time/week | 5.2 | 6.7 | 6.0 | 6.3 | ||
| 1–2 times/week | 66.2 | 74.6 | 75.2 | 75.7 | ||
| 3–6 times/week | 16.9 | 13.7 | 13.7 | 12.1 | ||
| Daily | 11.7 | 4.1 | 4.3 | 5.1 | ||
| Fish, % | 0.67 | 0.89 | ||||
| Never | 2.6 | 3.3 | 5.1 | 3.7 | ||
| Rare<1time/week | 45.5 | 41.9 | 41.0 | 39.9 | ||
| 1–2 times/week | 50.6 | 51.0 | 50.4 | 51.8 | ||
| 3–6 times/week | 1.3 | 1.3 | 0.9 | 1.7 | ||
| Daily | 0 | 2.6 | 2.6 | 2.9 | ||
| Fresh fruits, % | 0.15 | 0.56 | ||||
| Never | 1.3 | 1.6 | 0.9 | 2.2 | ||
| Rare<1 time/week | 10.4 | 6.6 | 11.2 | 9.3 | ||
| 1–2 times/week | 24.7 | 16.7 | 18.1 | 19.4 | ||
| 3–6 times/week | 24.7 | 22.8 | 20.7 | 25.3 | ||
| Daily | 39.0 | 52.2 | 49.1 | 43.8 | ||
| Raw green vegetables, % | 0.27 | 0.64 | ||||
| Never | 15.6 | 11.3 | 12.2 | 12.9 | ||
| Rare<1 time/week | 18.2 | 18.5 | 20.9 | 17.7 | ||
| 1–2 times/week | 32.5 | 28.9 | 35.7 | 31.3 | ||
| 3–6 times/week | 7.8 | 16.5 | 10.4 | 12.9 | ||
| Daily | 26.0 | 24.8 | 20.9 | 25.2 | ||
| Fruit juice, % | 0.84 | 0.67 | ||||
| Never | 2.6 | 2.7 | 3.4 | 2.0 | ||
| Rare<1 time/week | 13.0 | 10.3 | 8.5 | 11.3 | ||
| 1–2 times/week | 27.3 | 24.0 | 31.6 | 27.9 | ||
| 3–6 times/week | 16.9 | 20.8 | 22.2 | 22.0 | ||
| Daily | 40.3 | 42.2 | 34.2 | 36.8 | ||
| Burgers, % | ||||||
| Never | 41.6 | 35.8 | 0.41 | 15.7 | 20.3 | 0.64 |
| Rare<1 time/week | 42.9 | 50.5 | 59.1 | 57.6 | ||
| 1–2 times/week | 9.1 | 7.3 | 20.9 | 18.5 | ||
| 3–6 times/week | – | 2 | 3.5 | 2.1 | ||
| Daily | 6.5 | 4.4 | 0.9 | 1.5 | ||
| Antioxidants Eating Index (0–6), % | 4.3±1.1 | 3.9±1.3 | 0.004 | 4.0±1.2 | 3.9±1.1 | 0.27 |
| 0 | 0 | 0 | 0.01 | 0 | 0 | 0.71 |
| 1 | 5.2 | 1.3 | 1.8 | 2.4 | ||
| 2 | 6.5 | 6.3 | 8.8 | 7.8 | ||
| 3 | 24.7 | 15 | 25.7 | 22.8 | ||
| 4 | 26 | 25.8 | 28.3 | 26.3 | ||
| 5 | 26 | 33.9 | 28.3 | 28.8 | ||
| 6 | 11.7 | 17.7 | 7.1 | 12 | ||
p-Values retrieved from Pearson's chi square test.
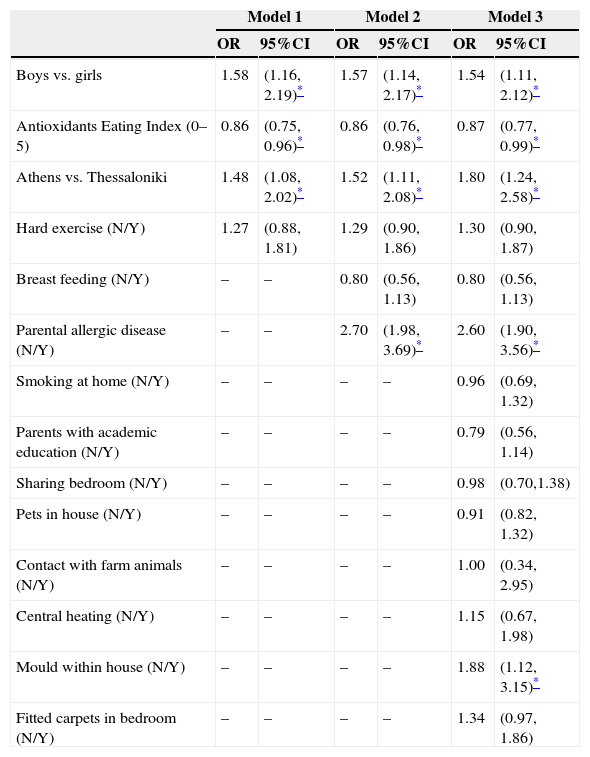
AEI score was inversely associated with lifetime asthma symptoms, after adjusting for gender, area of residence and exercise (model 1, Table 4). This association was also confirmed in further multi-adjusted analysis evaluating cities’ differences on other various environmental and lifestyle factors (models 2 and 3, Table 4). When the multi-adjusted analysis was focused on other allergic diseases and sensitisation, no association was found. AEI score was not associated with current asthma (OR=0.89, 95%CI 0.77, 1.03), lifetime rhinitis (OR=0.96, 95%CI 0.88, 1.05), current rhinitis (OR=0.93, 95%CI 0.85, 1.03), current eczema (OR=0.92, 95%CI 0.81, 1.04), lifetime eczema (OR=0.95, 95%CI 0.85, 1.06), and sensitisation to any allergen (OR=0.98, 95%CI 0.90, 1.07). Furthermore, logistic regression models were stratified by city of origin and sex of the children, but no differences on the effect of AEI on asthma symptoms were observed (data not shown, p for interaction(s) >0.7).
Results from multiple logistic regression models that evaluated lifetime asthma symptoms prevalence in 9–10-year-old children from the two major Greek cities, Athens and Thessaloniki.
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | OR | 95%CI | OR | 95%CI | |
| Boys vs. girls | 1.58 | (1.16, 2.19)* | 1.57 | (1.14, 2.17)* | 1.54 | (1.11, 2.12)* |
| Antioxidants Eating Index (0–5) | 0.86 | (0.75, 0.96)* | 0.86 | (0.76, 0.98)* | 0.87 | (0.77, 0.99)* |
| Athens vs. Thessaloniki | 1.48 | (1.08, 2.02)* | 1.52 | (1.11, 2.08)* | 1.80 | (1.24, 2.58)* |
| Hard exercise (N/Y) | 1.27 | (0.88, 1.81) | 1.29 | (0.90, 1.86) | 1.30 | (0.90, 1.87) |
| Breast feeding (N/Y) | – | – | 0.80 | (0.56, 1.13) | 0.80 | (0.56, 1.13) |
| Parental allergic disease (N/Y) | – | – | 2.70 | (1.98, 3.69)* | 2.60 | (1.90, 3.56)* |
| Smoking at home (N/Y) | – | – | – | – | 0.96 | (0.69, 1.32) |
| Parents with academic education (N/Y) | – | – | – | – | 0.79 | (0.56, 1.14) |
| Sharing bedroom (N/Y) | – | – | – | – | 0.98 | (0.70,1.38) |
| Pets in house (N/Y) | – | – | – | – | 0.91 | (0.82, 1.32) |
| Contact with farm animals (N/Y) | – | – | – | – | 1.00 | (0.34, 2.95) |
| Central heating (N/Y) | – | – | – | – | 1.15 | (0.67, 1.98) |
| Mould within house (N/Y) | – | – | – | – | 1.88 | (1.12, 3.15)* |
| Fitted carpets in bedroom (N/Y) | – | – | – | – | 1.34 | (0.97, 1.86) |
OR: odds ratio; 95%CI: 95% confidence interval.
The present work, under the context of the ISAAC II survey in Greece, suggested the protective role of foods that are rich in antioxidants to lifetime asthma. This seems to be the result of a combined high intake of vegetables, fruits and fish and a moderate to low intake of meat and burger. The association was strong and remained significant even after adjusting for other major potential confounders. Even though significant difference in asthma prevalence between the two cities did exist, the association was dominant in both cities. However, antioxidants food consumption was not related to allergic rhinitis, eczema and sensitisation.
Antioxidants are dietary factors that can mobilise the lung tissue response to oxidative stress reducing airway damage to reactive oxygen radicals. Vitamin C and E, carotenoids, flavonoids, selenium and zinc are major antioxidants that can be detected mainly in fruits and vegetables. In addition, fish are found to be not only enriched with n3 fatty acids but also with several types of endogenous antioxidants which can inhibit the oxidation of their lipids.24 Contrary to fish, meat and burgers are enriched with saturated and n6 polyunsaturated fatty acid which are major constituent of human inflammation cells. Diets marked by greater intakes of meat, poultry and fast foods during pregnancy and childhood have been related to unhealthy eating patterns and were associated with asthma and allergy outcomes.12,25–27 However, meat contains also selenium and zinc and others studies found no association with asthma symptoms.2,28 In this study, more than recommended meat intake was detected in 20% of the cases and the univariate analysis revealed an adverse effect of meat consumption on asthma prevalence in Athens; thus, the authors decided to use the increased meat intake as an unhealthy diet choice.
Previous observational studies have reported that children who daily consume antioxidant foods experienced lower wheezing episodes and allergic rhinitis.3,4,7–12 In the ISAAC phase I survey, the Gross National Product per capita (GNP) and the mean food intake rate of vegetables, wheat and vitamin A were related to lower asthma prevalence.3 The PIAMA birth cohort data analysis revealed that school age children, who were eating continuously high amount of fruits since infancy, had less asthma than children who refused.4 Moreover, high consumption of antioxidants was associated with higher pulmonary function and decreased chronic respiratory symptoms in children, especially those exposed to high levels of air or smoke pollutants.7,8,29,30 Fish consumption has been also related with lower asthma episodes in infancy and childhood.31–33 On the contrary, Cook et al. found no association between serum vitamin C levels and FEV1, but there was association between lung function and fruit consumption (a question arises if the blood levels reflect the consumed antioxidants).34 Moreover, the consumption of fruits in preschoolers did not improve asthma symptoms in later life,30 whereas the intake of fish oil in early childhood protected asthma at 18 months, but not at 5 years old.33,35
Discussing other allergic diseases, such as allergic rhinitis and eczema, antioxidants might affect immune function9 and allergy reactions.3,10,11,36 A survey of schoolchildren showed that asthma and allergic rhinitis were associated with reduced levels of serum antioxidant compared to healthy children.37 On the contrary, other studies failed to show any association between antioxidants and allergic rhinitis or sensitisation in accordance with the present study, whereas other reports suggested a negative association between the prevalence of eczema and beta-carotene, vitamin E or fish oil.5,6,17
Since the “nutrition hypothesis” has arisen, review and meta analyses failed to reveal clear association with the prevalence of asthma and allergies.9,13–17 In consequence, current nutrition epidemiology has the tendency to evaluate overall dietary habits as more realistic and closer to the truth, supporting a holistic approach of the impact of diet on human health. The Mediterranean diet score which is rich in fruits and vegetables and low in meat intake, was one of the first overall dietary indices developed and during the last few years, it has been evaluated against asthma with very promising results.12,28,38–40 Evidently, high level of adherence to the Mediterranean diet during pregnancy and early life protects against the development of childhood asthma.25,41
In our study, a healthy diet index was theoretically developed to act as an indicator of the antioxidants status with higher values suggesting a closer adherence to an “antioxidant” diet taking into account the oxidative effect of other food group like meat and burgers. Though a crude measure, it reflexes a simple holistic evaluation of antioxidant consumption. Detailed information on the consumption of legume and oil were lacking through the dietary questionnaire included in the ISAAC II survey, thus Mediterranean diet could not be assessed in our study, even though other data in Greece showed that families who highly consumed antioxidants were more obedient to the whole Mediterranean diet.42
Limitations of this studyThe attempt to obtain a scoring system measuring the influence of diet (a multifactorial habit) in asthma (a multifactorial disorder) shares great difficulties and the present work has all the methodological limitations of cross-sectional studies, i.e., the lack of causal relationships. The assessment of asthma by questionnaire is widely discussed in the ISAAC methodology that may over or under estimate the prevalence of the diseases. On the other hand, doubts arise whether dietary questionnaires can be a “mirror” of real nutrition in children. The dietary data was gathered by questionnaire which assesses the current diet habits at aged 9–10 years and possibly could not reflect the lifetime diet. In addition, children with oral allergy symptoms might avoid eating some specific foods like fruits and vegetables and thereby bias the results but no data on these could be collected through the ISAAC questionnaire. Furthermore, AEI has not been validated and did not include information on other dietary factors like whole-grains, legumes, butter or olive oil intake. Another limitation is that Body Mass Index could not be calculated and the influence of obesity was not evaluated. Lastly, participation rates in Thessaloniki were lower than in Athens which might be due to families with both non-Greek parents that were excluded, or/and high educated parents who were too busy to answer the questionnaire.
ConclusionUnder the context of the largest survey ever conducted regarding the epidemiology of asthma prevalence in children (the ISAAC phase II), this study revealed that antioxidant foods seem to be a non-pharmacological, protective means for reducing asthma symptoms irrespectively of atopy and heredity. Even though more evidence-based studies are needed, public health policies should pay close attention to the exploration of the role of the antioxidant-rich dietary pattern in children and their interrelationships on asthma in order to reduce the burden of the disease. Their role in other allergic diseases is still under consideration.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestThe authors have no conflict of interest to declare.