Respiratory syncytial virus acute bronchiolitis (RSV-AB) is a major cause of hospital admission among our infants. The immune and inflammatory mechanisms involved in the RSV-AB and factors influencing severity have not been clearly established, although an imbalanced Th1 and Th2 response seems to be crucial.
ObjectivesTo assess the local and systemic inflammatory response in RSV-AB. To find a possible marker of clinical severity and/or oxygen requirements.
Patients and methodsLevels of nine cytokines were measured in nasopharyngeal aspirate (NPA) and peripheral blood (PB) of 45 infants with RSV-AB and 27 peer controls, including IFNγ, TNFα, VEGF, interleukins 4, 6 and 10, and chemokines (IL-8 and macrophage inflammatory proteins 1-α and 1-β).
ResultsThe levels of the analyzed cytokines and chemokines were significantly higher in the NPA of RSV-AB group, with a decrease in IL-4/IFNγ ratio. IL-6 and MIP-1β levels in NPA were directly correlated to oxygen therapy. PB showed an increase in IL-8 and a decrease in MIP-1α and MIP-1β in the RSV-AB group (only MIP-1β associated to the need for oxygen therapy). No correlation was found between cytokines and chemokines levels in NPA and PB.
ConclusionsThis study shows that RSV triggers an inflammatory response fundamentally at the respiratory level, with scant systemic repercussion. This local response is characterized by an increase in Th1 and Th2 cytokines, although with a relative predominance of Th1. The determination upon patient admission of IL-6 and MIP-1β levels in NPA, and of MIP-1β in PB could help predict severe forms and the need for oxygenotherapy.
The airway inflammation observed in acute bronchiolitis due to respiratory syncytial virus (RSV-AB) is encompassed within a complex local response involving the participation of dendritic cells, lymphocytes, macrophages and particularly polymorphonuclear cells.1 These cells sequentially release cytokines, chemokines, proteases, reactive oxygen species (ROS) and other mediators during the evolution of acute bronchiolitis (AB).2 The existing data regarding the cytokine profile involved in the response to RSV are inconclusive.3–6
The severity of AB has been associated with an imbalance in the production of cytokines by the T helper cell subpopulations Th1 and Th2.7,8 The Th1 cytokines include interferon-gamma (IFNγ) and tumor necrosis factor-alpha (TNFα), which promote the host immune response to intracellular pathogens. On the other hand, Th2 lymphocytes produce preferentially interleukins 4 (IL-4), 5 (IL-5), 13 (IL-13), 10 (IL-10) and 16 (IL-16), chemokines such as interleukin 8 (IL-8/CXCL8), and macrophage inflammatory proteins (MIPs) 1α (MIP-1α/CCL3) and 1β (MIP-1β), which are seen to increase in situations of inflammation and/or allergy. The inflammatory response to RSV has fundamentally been investigated in respiratory samples such as nasal secretions and bronchoalveolar lavage (BAL), while the results obtained in peripheral blood (PB) are subject to controversy.4,9,10 Some authors have observed a prevalence of Th2 cytokines,9,11 while others have reported a prevalence of Th1 cytokines12 or a mixed Th1-Th2 inflammatory response.3,13 These discrepancies reflect the enormous complexity of the immune regulatory mechanisms that precisely modulate the balance between inflammatory and anti-inflammatory response.
The clinical manifestations of bronchiolitis are essentially of a respiratory nature with little systemic expression. This suggests that the inflammatory and immune responses to RSV are confined to the airway with a scant generalized inflammatory response, and explains the discrepancies between the levels of inflammatory markers measured at the respiratory level and in peripheral blood.
Since bronchiolitis is a potentially serious condition leading to hospital admission in infants during epidemic periods (autumn and winter months in the northern hemisphere), and is associated with wheezing for years after the infectious process, it would be interesting to identify biomarkers that are easy to measure, at both the respiratory level14–16 and in PB,17 and which are useful for diagnosing and predicting the course of the disease.
In this context, the primary aim of the present cross-sectional study is to analyze cytokine and chemokine levels in nasopharyngeal aspirate (NPA) and PB, in order to respectively assess the local and systemic response to AB in infants with RSV-AB, compared with healthy infants as controls. As secondary objectives, this study aims to evaluate the correlation between local and systemic responses to RSV, and to explore possible predictors of disease severity and the need for oxygen therapy.
MethodsParticipants and study designForty-five infants aged 1–11 months admitted to the Department of Pediatrics of Reina Sofía Children's University Hospital (Córdoba, Spain) with a first episode of acute bronchiolitis caused by RSV were recruited from 1st December 2009 to 31st March 2010. The inclusion criteria were a prodromal history consistent with viral upper respiratory tract infection (cough, coryza, rhinorrhea), increased respiratory effort (tachypnea, intercostal retractions or use of accessory muscles) and/or abnormal auscultation findings (wheezing or crackles). Patients were excluded from the study in the presence of one or more of the following: symptoms for longer than five days, premature birth, low weight (≤P5) or overweight (≥P85), a history of chronic or congenital diseases of the lungs, heart or nervous system, immune deficiency or previous treatment with palivizumab or corticosteroids. Infants with RSV-negative bronchiolitis were likewise excluded.
The control group comprised 27 healthy infants under 12 months of age, without respiratory tract illness during the previous 30 days or any history of hospital admission due to bronchiolitis or wheezing, and recruited during the same period as the bronchiolitis group. These infants came from the Department of Surgery, where a preoperative study, including blood tests, was indicated for elective surgery. The study protocol was previously approved by the local Ethics Committee.
At the time of enrolment, the parents or legal guardians were asked to sign the informed consent form and to answer questions referred to demographic characteristics and the presence of risk factors associated with bronchiolitis, including exposure to tobacco smoke, familial atopy, atopic dermatitis and breastfeeding history.
Severity assessmentThe severity of bronchiolitis was assessed upon admission and daily during hospitalization by a single investigator, using a modified severity score for bronchiolitis18 that includes wheezing/crackles, intercostal retraction, respiratory frequency, heart rate and cyanosis. Moderate to severe disease was defined as a score of 4 or greater, in order to divide the patients into two severity subgroups. Simultaneously, the RSV-AB group was subdivided depending on the oxygen needs during hospital admission. Oxygen therapy was indicated in the case of persistent SatO2≤92%.
Samples collectionSamples were collected on the first day after admission. After instilling 3ml of saline solution in each nostril, NPA were collected in a sterile mucous trap applying mild suction. The samples were centrifuged and the supernatant was divided into aliquots. Blood samples were subsequently collected using an indwelling venous line to draw a 3-ml sample into a tube containing EDTA. After centrifugation at 3500×g for 10min, plasma and the buffy coat were removed into different Eppendorf tubes. The serum and NPA supernatant samples were then frozen at −82°C until cytokine analysis.
Cytokine/chemokine analysisBiomarkers were measured in NPA and plasma samples with a Luminex® xMAP™ multiplexing system using Labscan™ 100 technology and LINCOplex assay kits, based on the use of fluorescently-dyed beads conjugated with monoclonal antibodies specific for nine target cytokines and chemokines: IL-4, IL-6, IL-10, TNFα, IFNγ, vascular endothelial growth factor (VEGF), IL-8 (CXCL8), MIP-1α (CCL3), and MIP-1β.
Statistical analysisData were expressed as the mean±standard deviation (SD) or median (interquartile range) for quantitative variables, while qualitative variables were reported as absolute and relative frequencies. In all cases statistical significance was considered for p<0.05 with two-tailed contrasts.
Normal data distribution was assessed by the Shapiro–Wilk test, and the homogeneity of variances was estimated using the Levene test. Comparisons of means between groups in the case of continuous variables with a normal distribution were carried out with the Student t-test for unpaired samples, while the Mann–Whitney U-test was used for variables with a non-normal distribution. Categorical data were analyzed using the chi-squared test or Fisher exact test.
Since there were differences in the mean age of the patients with bronchiolitis and the controls, the two groups were compared using analysis of covariance (ANCOVA) adjusted for age, and Sidack's correction. Correlations between variables were assessed using Spearman's rho analysis. The Statistical Package for Social Science (PASW Statistic 18; SPSS, Chicago, IL, USA) was used throughout.
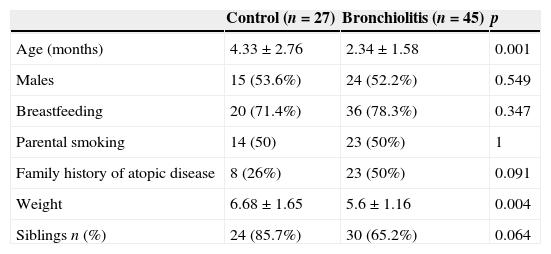
ResultsForty-five infants with RSV-AB were included in the study (17 mild and 28 moderate-severe cases according to the severity score definition of <4 or ≥4, respectively) along with 27 controls. Twenty-one infants (47%) required oxygen therapy during hospitalization. The baseline characteristics of the infants included in the study are shown in Table 1. There were no differences in terms of sex, type of feeding or exposure to tobacco smoke between the groups. The only significant difference between them corresponded to age and, consequently, body weight.
Demographic characteristics of the infants with RSV-AB and controls.
| Control (n=27) | Bronchiolitis (n=45) | p | |
|---|---|---|---|
| Age (months) | 4.33±2.76 | 2.34±1.58 | 0.001 |
| Males | 15 (53.6%) | 24 (52.2%) | 0.549 |
| Breastfeeding | 20 (71.4%) | 36 (78.3%) | 0.347 |
| Parental smoking | 14 (50) | 23 (50%) | 1 |
| Family history of atopic disease | 8 (26%) | 23 (50%) | 0.091 |
| Weight | 6.68±1.65 | 5.6±1.16 | 0.004 |
| Siblings n (%) | 24 (85.7%) | 30 (65.2%) | 0.064 |
Qualitative variables are expressed as absolutes frequencies (percentages) and continuous variable as the mean±SD. Chi-squared, Fisher's exact and Mann–Whitney U tests were used to determine differences between groups. p≤0.05 was considered to be significant.
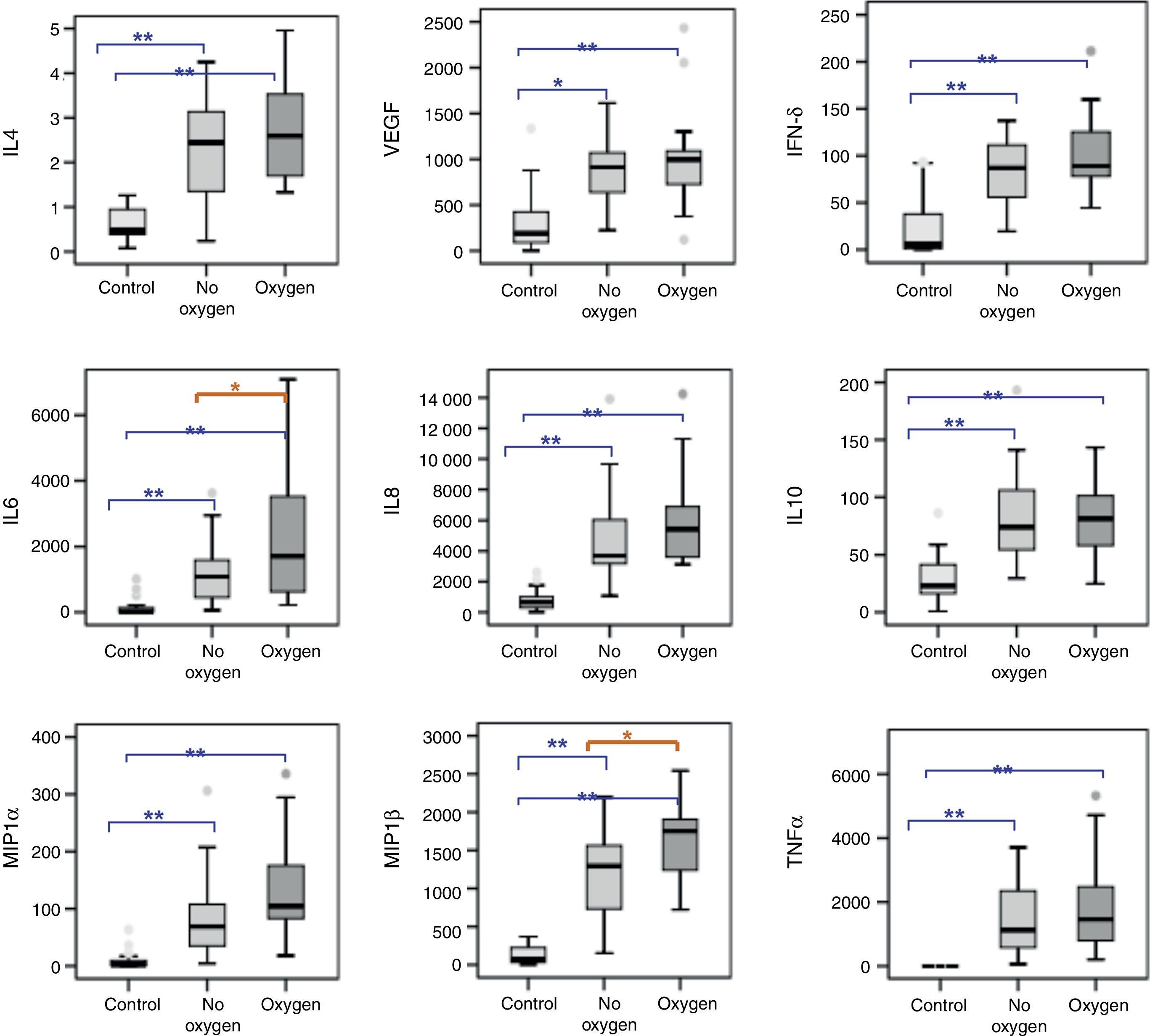
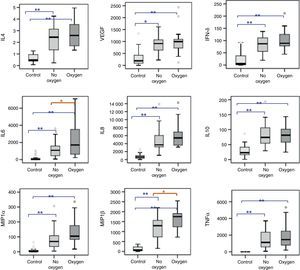
After adjusting for age, the cytokine and chemokine levels were seen to be significantly increased in NPA of the infants admitted with RSV-AB. The analysis of subgroups revealed no differences in the respiratory levels of cytokines and chemokines according to the severity of bronchiolitis, although significant IL-6 and MIP-1β elevations were recorded in NPA of those infants with AB requiring oxygen versus those who did not require oxygen therapy (Fig. 1).
Cytokines and chemokines levels (pg/ml) in NPA of healthy controls (N=27) and infants with RSV-AB (N=45) classified according to oxygen requirements. Statistical significance between control and bronchiolitis groups on applying ANCOVA (analysis of covariance) after adjusting for age. Data are expressed as the mean±SEM. *p<0.05; **p<0.001. IL-4, interleukin 4; VEGF, vascular endothelial growth factor; IFNδ, interferon-gamma; IL-6, interleukin-6; IL-8, interleukin-8; IL-10, interleukin-10; MIP-1α, macrophage inflammatory protein-alpha; MIP-1β, macrophage inflammatory protein-beta; TNFα, tumor necrosis factor-alpha.
The IL-4/IFNγ ratio (indicative of Th1/Th2 response) in the bronchiolitis group was significantly reduced with respect to the control group (0.02±0.005 vs. 2.57±7.41, respectively; p=0.009). There were no differences in IL-4/IFNγ ratio according to the severity score (mild: 0.026±0.006 vs. moderate-severe: 0.027±0.006; p=1) or oxygen requirements (without O2: 0.026±0.002 vs. with O2: 0.027±0.004; p=0.996).
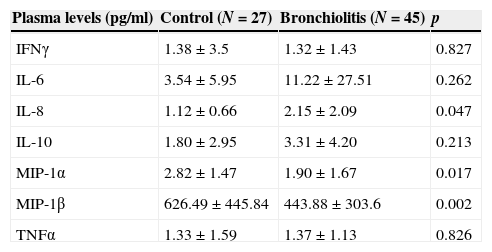
Cytokines and chemokines levels in plasmaThe plasma measurements showed a significant increase in IL-8 and a decrease in the concentrations of chemokines MIP-1α and MIP-1β (Table 2) among the infants with RSV-AB. The concentrations of the rest of cytokines were similar in both groups. There were no significant differences in cytokine and chemokine levels according to either the severity score or the need for oxygen, except in the case of MIP-1β, which was significantly lower in patients requiring oxygen therapy or with a moderate-severe severity score versus the mild presentations and controls (RSV-AB with O2: 365.4±240.9pg/ml; RSV-AB with severity score <4: 415.2±283.7pg/ml, vs. control: 626.49±445.8pg/ml; p=0.002 and p=0.006, respectively).
Plasma concentrations (pg/ml) of chemokines and cytokines in infants with RSV-AB and controls. IFNγ, interferon-gamma; IL-6, interleukin-6; IL-8, interleukin-8; IL-10, interleukin-10; MIP-1α, macrophage inflammatory protein-alpha; MIP-1β, macrophage inflammatory protein-beta; TNFα, tumor necrosis factor-alpha.
| Plasma levels (pg/ml) | Control (N=27) | Bronchiolitis (N=45) | p |
|---|---|---|---|
| IFNγ | 1.38±3.5 | 1.32±1.43 | 0.827 |
| IL-6 | 3.54±5.95 | 11.22±27.51 | 0.262 |
| IL-8 | 1.12±0.66 | 2.15±2.09 | 0.047 |
| IL-10 | 1.80±2.95 | 3.31±4.20 | 0.213 |
| MIP-1α | 2.82±1.47 | 1.90±1.67 | 0.017 |
| MIP-1β | 626.49±445.84 | 443.88±303.6 | 0.002 |
| TNFα | 1.33±1.59 | 1.37±1.13 | 0.826 |
Statistical significance between control and bronchiolitis groups on applying ANCOVA (analysis of covariance) after adjusting for age. Data are expressed as the mean±SEM.
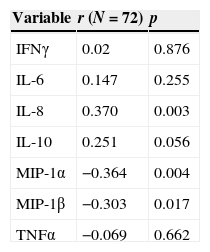
No significant correlation was observed between the cytokine levels measured in NPA and plasma. Low significant associations were noted between the chemokine concentrations in plasma and respiratory secretions, with a direct correlation in the case of IL-8 and an inverse correlation (elevated in NPA and diminished in plasma) in the case of MIP-1α and MIP-1β (Table 3).
Correlation between cytokine levels in nasal secretions and plasma. IFNγ, interferon-gamma; IL-6, interleukin-6; IL-8, interleukin-8; IL-10, interleukin-10; MIP-1, macrophage inflammatory protein-1; TNFα, tumor necrosis factor-alpha.
| Variable | r (N=72) | p |
|---|---|---|
| IFNγ | 0.02 | 0.876 |
| IL-6 | 0.147 | 0.255 |
| IL-8 | 0.370 | 0.003 |
| IL-10 | 0.251 | 0.056 |
| MIP-1α | −0.364 | 0.004 |
| MIP-1β | −0.303 | 0.017 |
| TNFα | −0.069 | 0.662 |
r, Spearman's rho coefficient.
The pathogenesis of RSV-AB is characterized by the combination of direct cytopathic action of the virus upon the bronchial mucosa and the associated complex local inflammatory reaction, with the sequential release of cytokines, chemokines, proteases and reactive oxygen species, among other mediators.2 A number of studies15,19–21 have found the levels of cytokines and chemokines in NPA to show a good correlation with the concentrations found in bronchoalveolar lavage (BAL). This observation, and the fact that nasopharyngeal aspiration is easy and non-invasive, define NPA as the samples of choice for studying airway inflammation in acute bronchiolitis.
In the present study all the cytokines and chemokines analyzed in infants admitted due to RSV-AB were found to be increased in NPA, although without differences between subgroups according to the severity score. In contrast, significant IL-6 and MIP-1β elevations were observed in NPA among those infants with AB requiring oxygen therapy. This finding, and the detection of very low plasma concentrations of cytokines and discrete modifications in chemokines, suggest that the immune and inflammatory response to RSV occurs at a local level within the airway, with scant systemic repercussion, as has been previously reported.22
Controversy persists regarding the cytokine profile generated in response to RSV infection – with the existing data being inconclusive in this respect.3–6 There have been reports of a predominance of Th2 cytokines,9,11 of Th1 cytokines,12 and of a mixed Th1-Th2 response pattern.3,13 These discrepancies may be due to factors dependent upon the design of the studies, such as the severity score used, the characteristics of the infants (age, need for admission or oxygen therapy), the timing of the sample collection, or the possible influence of comorbidities.23,24 Another aspect to be taken into account is the possible influence of environmental factors such as tobacco smoke25 and genetic parameters26–28 upon the production of cytokines and chemokines.
In the present study, although we observed elevations of all the cytokines in NPA, the IL-4/IFNγ ratio was significantly lowered among the infants with RSV-AB, thus indicating a relative predominance of Th1 responses in this patient group versus the controls. Other authors have published similar results.12,29 However, these data are in contrast to the observations of other investigators such as Legg et al.,4 who recorded a predominance of Th2 responses (increased IL-4/IFNγ ratio) in infants who developed AB versus those who only presented upper airway RSV infection. In turn, Bermejo-Martín et al.,30 observed a relative predominance of Th2 responses to RSV on comparing different Th1 and Th2 cytokine ratios other than IL-4/IFNγ in infants with AB. A differentiating element in our study with respect to the above commented publications is the fact that the latter included older bronchiolitis patients. This might indicate that the Th2 responses increase with age,31 and underscores the relevance of a relative predominance of Th1 responses in younger infants, since these may constitute the main response to RSV infection in such patients. While the mean age of the RSV-AB patients in our study was under three months, the age of the controls was slightly greater, since infants requiring admission due to bronchiolitis tend to be comparatively younger. On the other hand, the ethical problems associated with the conduction of studies with healthy infants5,7,15,30 also causes the control group to be older than the bronchiolitis group. In any case, our statistical analysis was adjusted for age in order to avoid any possible influence of this parameter upon the results obtained.
The pathogenesis of AB may be characterized by an exaggerated inflammatory response mediated by Th1 cytokines32 that would prevail over the control mechanisms designed to correct excessive inflammation. Studies in murine models have evidenced the dual role of IFNγ, which on the one hand limits viral replication and counters the action of Th2 cytokines, and on the other is associated to airway obstruction33 secondary to stimulation of the innate immune response and inhibition of the adaptive response – generating inflammation with acute and chronic bronchial damage, and allowing recurrent viral infection34 by evading the memory defence response.
There is also controversy as to whether29,35–37 or not20 the cytokine profile varies according to the infective virus involved. In order to avoid this confusion factor, we only included previously healthy infants born to term and admitted to a single hospital center due to AB with positive RSV antigen titers, during the same epidemic period, and with sampling performed within the first 24h after admission.
In coincidence with other authors,20,38,39 we found the severity of bronchiolitis to be more closely correlated to chemokine elevations than to cytokine increments, since we only recorded a significant correlation between the need for oxygen therapy during admission and an increase in IL-6 and MIP-1β levels in NPA. Garcia et al.,20 found a correlation between the oxygen needs and the MIP-1α (direct) and IFNγ (inverse) levels. Similar findings have been reported by Semple et al.,15 although it is not clear whether this has an impact upon the global severity of the condition (symptoms score or days of admission). In any case, these data must be interpreted with caution, since the first of the aforementioned studies did not analyze the severity parameters (oxygen therapy and duration of admission) according to patient age, despite the inclusion of infants under two years of age. In turn, in the second study, the patients requiring oxygen therapy were older than those who did not. Once again, age appears to be a determining factor in the analysis of cytokine levels.
The above findings are in contrast to those of Bennet et al.,40 who recorded an inverse correlation between the levels of IL-6, MIP-1β, IL-8 and IL-10 in NPA and the severity of AB, suggesting that they act as protective factors against hypoxia. However, this study included infants with AB due to RSV and other viruses which could produce different cytokine release profiles.
We also recorded increased levels of vascular endothelial cell growth factor (VEGF) in NPA, although without significant differences according to the need for oxygen therapy or the severity score. RSV and hypoxia are potent inducers of VEGF,41 which stimulates angiogenesis in the inflammatory zone, as well as macrophage and granulocyte chemotaxis, and increases the permeability of the bronchiolar respiratory epithelium.
We analyzed cytokines and chemokines in parallel in plasma to assess the systemic repercussions of the inflammatory response to RSV. Very low cytokine and chemokine levels were found (with the exception of MIP-1β), thereby indicating a scant systemic response to the virus. No significant differences in the levels of cytokines were observed with respect to the controls, although in contrast the chemokines revealed increased IL-8 and lowered MIP-1α and MIP-1β concentrations in the infants with RSV-AB. This could be the result of the migration of macrophages (the main producers of MIP) through leukodiapedesis from the capillaries toward the airway mucosa, where an increased local consumption of MIP-1α and MIP-1β would result in a decrease in their plasma levels. On comparing the plasma levels of these chemokines according to the severity, we only found a significant decrease in MIP-1β in the patients with AB requiring oxygen therapy or with a moderate-severe disease score, compared with mild RSV-AB. The findings at the respiratory level20 and in peripheral blood42 suggest that the MIP-1β production capacity could be related to the pathogenesis of the disease.
No modification was observed in the IFNγ levels in peripheral blood among the infants with RSV-AB, thus supporting the idea that the infection and subsequent inflammation generated by RSV are limited to the respiratory epithelium, since RSV is rarely isolated from blood44 or extrapulmonary tissues.45,46 Other authors have reported increased7 or diminished plasma IFNγ levels.5,17,43 These contrasting observations among studies could be explained by the methodological differences referred to above.
In coincidence with other investigators,22,30,39 we found no association between the levels of each cytokine in NPA and in plasma. In contrast, we observed a significant (but weak) association between the levels of chemokines in plasma and in respiratory secretions. Specifically, a direct correlation was recorded in the case of IL-8, and an inverse correlation in the case of MIP-1α and MIP-1β (elevated in NPA and diminished in plasma). This supports the existence of two almost independent compartments in the immune and inflammatory response to RSV, interconnected by chemokines. The latter constitute key elements whose activation induces migration of the cells from the bloodstream toward the affected compartment, i.e., the bronchioles.
In any potentially serious condition such as bronchiolitis, it is of interest to identify potential prognostic indicators. In this context, we recorded an increase in IL-6 and MIP-1β in NPA and a decrease in MIP-1β in plasma among infants with AB requiring oxygen therapy during admission, although we found no correlation with the bronchiolitis severity score. We therefore consider it interesting to reorientate the assessment of AB severity criteria, adding more objective measures such as transcutaneous oxygen saturation to the clinical scores, which are characterized by a certain amount of subjectivity.
The major strength of our study is the fact that the number of participants was obtained from among all the patients admitted due to AB with positive RSV testing in the same epidemic period and in a single hospital center. A limitation of the study is the small size of the sample and thus of the subgroups, due to the ethical limitations in recruiting the control group. This explains the case/control ratio of 1.6/1.
Overall, the data obtained indicate that the immune and inflammatory responses to RSV infection are essentially circumscribed to the airway, with a non-specific elevation of all the biomarkers measured in nasopharyngeal aspirate, although with a relative predominance of the Th1 response. In contrast, with the exception of the activation of chemokines, few inflammatory repercussions were recorded at a systemic level.
The finding of an elevation of IL-6 and MIP-1β in respiratory secretions and of a decrease in plasma MIP-1β, associated with the need for oxygen therapy and/or increased severity of RSV-AB, define these molecules as potential markers of disease severity or at least of hypoxemia. Consequently, the determination of these biomarkers in NPA and/or serum could be considered in clinical practice. Further multicentre and prospective trials are required to clarify these issues.
Ethical disclosuresProtection of human subjects and animals in researchProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients mentioned in the article. The author for correspondence is in possession of this document.
Conflicts of interestThe authors declare that they have no conflicts of interest in relation to this article.
This study was supported in part by a grant from the Spanish Society of Pediatric Pulmonology (SENP) in 2009. This work was awarded with the prize to the best oral communication at the XXXVIII annual congress of the Spanish Society of Pediatric Allergology, Asthma and Clinical Immunology (SEICAP).