We have previously described gastro-allergic Anisakiasis as a well defined clinical entity in which the live third stage larva of Anisakis simplex ( A.s. ) produces acute IgE-mediated allergic hypersensitivity symptoms frequently accompanied by abdominal symptoms 1-3 . The resulting cutaneous symptoms like urticaria or angioedema are mainly self-limited in less than 24 hours. Only rarely the patients with this entity complained in the acute phase of longer lasting allergic symptoms up to 14 days 4 . The nematode does not survive more than a few days in humans and the rare known chronic complications are due to a local inflammatory reaction against dead material. Followup of these patients with acute gastro-allergic Anisakiasis with dietary advice show neither further fish-related symptoms nor cutaneous symptoms.
On the other side chronic urticaria (CU) has been associated with the detection of IgE-antibodies against A.s. 5,6 . The implication of these IgE-antibod-ies in chronic urticaria as well as possible dietary advice are still controversial.
The interpretation of detectable serum specific IgE to A.s. is limited because of a long lasting memory after an acute parasitism. Even patients with acute parasitism of gastric or possibly other intestinal location produce detectable IgE antibodies in the absence of allergic cutaneous hypersensitivity symp-toms 7,8 . This leads to a high prevalence of detectable IgE antibodies against this nematode in our region. On the other side the induction of the Th2 lymphocyte subset in helminthosis results in stimulation of both IgG4 and IgE 9 . It has been reported that the parallel production of IgE and IgG4 reflects the competition for the same antigenic target and the ratio of IgE to IgG4 has been proposed in determining the expression of allergic symptoms 1,11 . We therefore studied the relationship between this nematode and chronic urticaria (CU) as well as the clinical usefulness of the measurement of specific in chronic urticaria.
For this purpose we first estimated the prevalence of specific IgE against A.s. in patients with chronic urticaria. We then analyzed the clinical response of CU patients to a two-months diet without fish, and finally we compared the improvement rate, other clinical data and specific immunoglobulins in sensitized patients with and without detectable specific IgG4.
METHODS
Study design and patient selection1. 135 consecutive adult patients with CU were studied for sensitization to A.s. with means of serum specific IgE and Skin Prick Test (SPT). Patients were included if they displayed persistent or frequently recurrent wheals for at least two months. Those patients with detectable food or drugs as trigger factors as the only causes of recurrent symptoms were excluded. A complete laboratory investigation was performed in order to look for other diseases or situations that are associated with chronic urticaria. (13) C-urea breath test was performed in 62 patients in order to assess infection by Helicobacter pylori . The prevalence of detectable specific serum IgE against A.s. was estimated independently of other results and compared with known data about prevalence of sensitization to this parasite in healthy subjects in our area.
2. Then, out of the whole group, 76 patients were put on a rigorous diet without any fish or seafish for two months, and we analyzed the clinical response: Clinical improvement was defined as a significant de-crease in the number of antihistamine tablets taken or disappearance of urticarial lesions. Group A was comprised of 65 patients with specific IgE > 0,7kU/l and a positive SPT and in the control group B 11 patients were included who were not sensitized to A.s. (negative SPT and specific IgE < 0,35 kU/l). We compared the prevalence of patients with a clinical improvement in both groups. As CU is a probably multifactorial cutaneous expression, the studied groups were evaluated independently of other factors known to be associated with CU.
3. In a third step and in order to elucidate a possible diagnostic value for specific IgG4, we divided patients of group A (chronic urticaria and sensitization to A.s. ) into groups C and D (detectable and no detectable specific IgG4, respectively) and compared them with respect to age, duration of symptoms, specific IgG and IgE against A.s. as well as total IgE. Patients were asked for previous episodes of acute urticaria/angioedema associated with intake of fish and the prevalence of previous episodes was compared in both groups. Patients with previous urticarial reactions without evident trigger factor were excluded in this analysis. The prevalence of rhinoconjunctivitis or asthma and a positive (13) C-urea breath test were also compared.
Laboratory
Measurement of specific IgE, IgG and IgG4 against A.s. was performed with CAP-FEIA (Pharmacia, Uppsala, Sweden). Total IgE was assessed by IMx-Method Abbot Diagnostics, Chicago, IL, U.S.A.). Specific IgE was considered positive at > 0,35 kU/l, specific IgG was considered positive at > 2 mg/l and specific IgG4 was considered positive at > 150 g/l.
StatisticsAll statistical analysis was performed using SPSS 8.01 for WINDOWS (SPSS Inc., 1989-1998).
After analysing each variable with Kolmogoroff-Smirnoff-Test for Normal distribution, mean values and standard deviation were obtained for age and duration of symptoms and compared by Student’s t-test. Median values and interquartile ranges were obtained for total IgE and specific IgE, IgG and IgG4 against A.s. and compared by and Mann-Whitney. Chi-Square Test and Fisher’s Exact Test were used in order to compare in the different groups the clinical outcome, the prevalence of previous acute fish-related urticaria, rhinoconjunctivitis or asthma and positive (13) C-urea breath test.
RESULTS
Estimating the prevalence of sensitization to A.s. in chronic urticaria135 consecutive adult patients with chronic urticaria had a mean age of 41,5 ( ± 15,4) years old and a sex distribution of 91 women/44 male. We detected IgE-antibodies against A.s. in 52,6 % (n = 71) with a median of 8,6 (IQR 2,4-23) kU/l (considering only detectable values). In contrast, previous estimations of sensitization to A.s. in healthy controls and blooddonors in our area showed only a prevalence between 16 % and 20 % in adults 4,9 .
Other significant results, independently of the sensitization status, in routine examination of these patients were infection by Helicobacter pylori in 48/62 patients (77,4 %), intestinal protozoa ( Giardia lamblia , Blastocystis hominis , Iodamoeba butschlii or Entamoeba coli ) in 12 patients, chronic hepatitis B or C in 4 patients, thyroid dysfunction in 4 patients (one with detectable antithyroid antibodies).
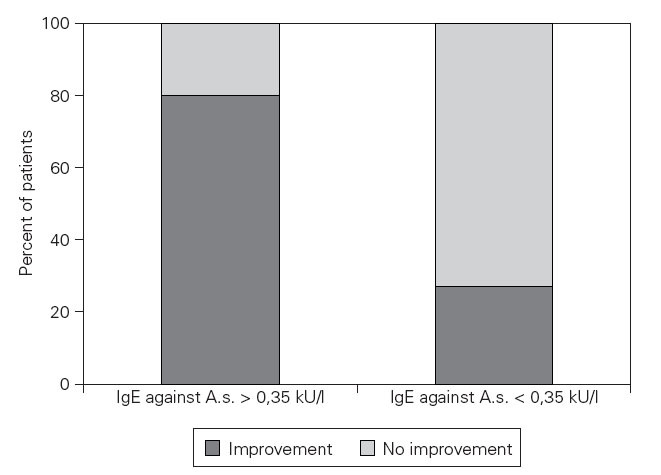
Dietary-based clinical response in patients with and without sensitization to A.s.In the sensitized group A, 52 patients experienced a clinical improvement and 13 patients did not improve after a two months diet. In the non-sensitized control group (B) 3 patients experienced a clinical improvement and 8 patients did not improve (Fisher’s Exact Test: p = 0,001) (fig. 1).
Analyzing specific IgE, IgG and IgG4In the group of 65 sensitized patients (group A) median IgG4 was 325 ( p25 0 and p75 903) g/l and was not detectable in 22 patients. Specific IgG4 was not detectable in any of the 11 non sensitized patients (group B).
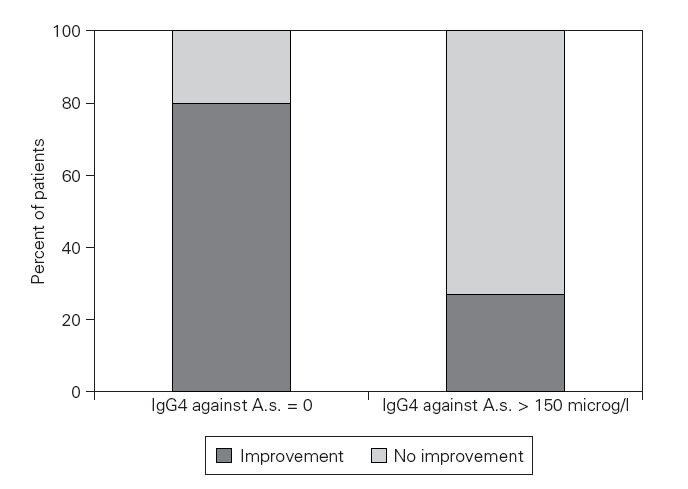
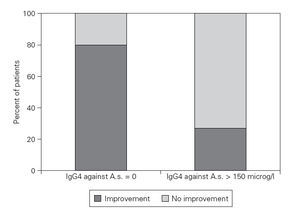
38 of 43 patients with detectable specific IgG4 (group C) showed a clinical improvement compared to only 14 of 22 patients without detectable IgG4 (group D) (Fisher’s Exact Test: p = 0,02). An optimal predictive cut-off level was found at 175 g/l for specific IgG4 with a ratio of 37/40 compared to 15/25, respectively (p = 0,004) (fig. 2).
Figure 1.—Specific IgE dependent improvement rate after a diet without fish. Impovement rate. Impovement rate after a 2 months diet without any fish or seafish in patients with chronic urticaria. The patients sensitized to Anisakis simplex experienced a significant clinical improvement in a higher percentage compared to patients without detectable IgE antibodies against A.s. (Fisher’s Exact Test: p = 0,001).
Mean age was similar in both groups with 43,6 ± 15,4 years in group C and 45,9 ± 13,2 years in group D. Mean duration of symptoms was lower with 6,7 ± 4,8 months in group C than in the group without detectable IgG4(group D: 10,1 ± 6,7 months, p = 0,04).
Figure 2.—Specific IgG4 dependent improvement rate after a diet without fish in Anisakis simplex sensitized patients. Improvement rate after a 2 months diet without any fish or seafish of patients with chronic urticaria. The patients with detectable specific IgG4 against Anisakis simplex experienced a significant clinical improvement in a higher percentage compared to patientes without detectable IgE antibodies against A.s. (Fisher’s Exact Test: p = 0,02).
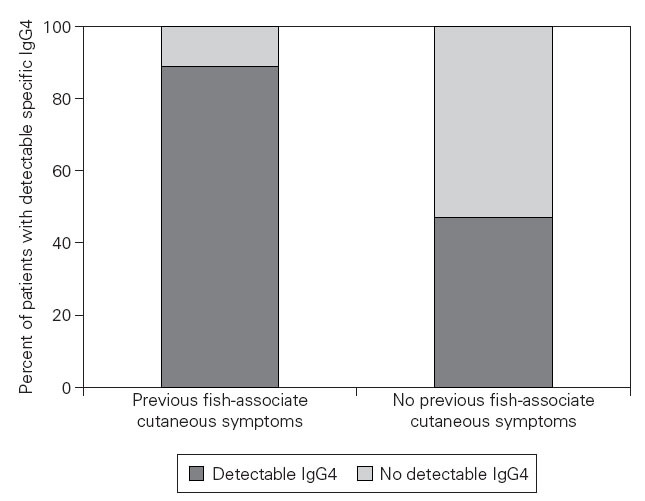
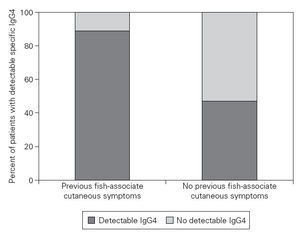
Figure 3.—Relationship between previous gastro-allergic anisakiasis and detectable specific IgG4 values. It can be assumed that our patientes with a history of previous fish-associated cutaneous symptoms suffered a previous episode of gastro-allergic anisakiasis. These patients have a higher probability of displaying detectable specific IgG4 values (Fisher’s Exact test: p = 0,03).
Median specific serum IgG was higher in group C than in group D (11,6; IQR 7,6-16,6 versus 6,1; IQR 5,5-8,6 mg/l, p < 0,0005). Also specific IgE was higher in group C (20,0; IQR 6,8-37,0 versus 4,5; IQR 1,7-14,3 IU/l, p < 0,0005). Total IgE was not significantly higher in group C than in group D (173; IQR 99-291 kU/l versus 133; IQR 72-205 kU/l, p = 0,1).
As it is already known that immunoglobulin levels vary after a parasitic episode depending on the time elapsed 2,13 , we crossmatched patients from group C and D in order to get two groups with chronic urticaria of similar duration. The results for specifoc IgG and IgE, as well as the improvement rate do not change significantly.
Eight of nine patients with previous fish-associated cutaneous symptoms displayed detectable specific IgG4, compared to 15 of 32 patients who denied any neither previous fish-associated symptoms nor acute urticaria (p = 0,03) (fig. 3). Helicobacter-pylori infection or rhinoconjunctivitis/asthma did not significantly predispose the patients to belong to one of both groups.
DISCUSSIONChronic urticaria is a frequent and disabling illness ocurring worldwide in 0.1 % of the population 14 . Otherwise a recent survey in our area estimates the prevalence of CU about 2,5-2,9 % 15 . CU has been classified in physical and idiopatic urticaria. Only in recent years chronic idiopathic urticaria has been labeled autoimmune in a high percentage of cases 14 . CU induced by infections has been proposed over the last 30 years but is anecdotical and the mechanisms are not clarified. Although still controversial, Helicobacter pylori seems to have an indirect role in some patients with chronic urticaria and parasitic disease has not been confirmed to play a role in this en- 14,16,17 . Thus, up to now no specific microbial antigen has been identified that precipitates episodes of hives in patients with chronic urticaria.
In this study we describe various results that underline a possible causal relationship between the ubiquitous nematode A.s. in a high proportion of patients with CU in our area.
First, the high prevalence of detectable IgE antibodies against A.s. in patients with chronic urticaria demonstrates a clear association between these antibodies and at least a subgroup of patients with this disease. In an acute episode of urticaria a detailed medical history is mostly sufficient to suspect a live third stage larva to be responsible, and a complementary examination with skin prick tests and serial specific and total IgE confirms the diagnosis of gas-tro-allergic Anisakiasis without the need of a gastroscopic confirmation, which will only be performed according to the requirements in the emergency 1,2,4 . Cross-reactivity has been suspected as an explanation for detectable IgE antibodies against A.s. and has been demonstrated in vitro with house dust mites, cockroaches, chironomids, crustaceans and other parasites of the nematode order 18-20 . The clinical importance of such cross-reactivity is still uncertain and it does not explain the high ratio of patients with chronic urticaria and presence of detectable specific IgE against A.s. , as only anecdotical reports have associated chronic urticaria with house-dust-mite allergens or parasites and none of the possible cross-re-acting agents have been reported to cause chronic urticaria on a wide scale 21-23 . Thus, cross-reactivity can not be made responsible for the high prevalence of theses antibodies. The fact that in our area 15-20 % percent of the normal population display specific IgE against A.s. reflects that a high proportion of these had previous, perhaps subclinical episodes of acute parasitism with production of long lasting IgE antibodies. However in patients with chronic urticaria it can not be ruled out that the detection of specific IgE antibodies represents a marker of a causal relationship between A. s and CU, more if we take into account that we demonstrate the prevalence of these antibodies to be much higher in chronic urticaria patients than in healthy individuals.
Patients with detectable specific IgE anti- Anisakis antibodies experienced a significant clinical improvement after a two months diet without any fish. If we compare this group with patients without sensitization to A.s. who only improved in a minority of cases, we deal with two different groups of CU patients and a different clinical outcome. It could be possible that our CU patients with detectable specific IgE-antibod-ies have a CU disease of shorter duration, but our assessment by cross-matching patients with respect to the duration of symptoms showed that patients improved in the sensitized group irrespective of the previous duration of symptoms. As we have no control group of patients sensitized to A.s. without any dietary recommendations, we cannot confirm the real beneficial effect of our dietary recommendations in sensitized patients, but we get a clear-cut separation of CU patients in different groups. These results underline again a possible causal association between A.s. and CU.
If we then analyzed only sensitized patients with respect to the clinical outcome after a diet we found a positive relationship of high specific IgG4values and clinical improvement. We have previously shown that an acute parasitism by A.s. is accompanied by the production of specific immunoglobulin antibodies of all classes including IgG4. If we consider patients with chronic urticaria who remember previous fish-associated acute urticaria or angioedema we find that nearly all these patients display detectable specific IgG4. This supports the hypothesis that detectable IgG4 against A.s. could be a marker of a previous acute parasitism. Here IgG4levels seem to be a prognostic marker with respect to dietary recommendations. Alltogether we find again arguments that implicate A.s. in CU at least in the group of patients with detectable specific IgG4.
Even in the absence of previous episodes of acute urticaria, about 30 % of chronic urticaria patients with IgE antibodies against A.s. display detectable IgG4 with a mainly good prognosis. This can be explained by the fact that previous acute parasitism could have been subclinical or with only digestive symp- 1,22,25 . Our results indicate that the detection and the magnitude of specific IgG4 levels are partly dependent on the other specific immunoglobulin levels. With respect to patients with no detectable IgG4, we have thus two possibilities to explain the association of low or undetectable specific IgG4 levels with a poorer prognosis.
First, lower levels of immunoglobulins could be in accordance with a longer time interval between a previous parasitism and their analysis. Therefore we crossmatched patients with respect to the duration of CU, but obtained similar results. Thus the detected immunoglobulin levels could simply be a marker of a previous parasitism (prior to the initiation of CU) and thus without any clinical relevance. This would be in accordance with the fact that in the group of CU patients we would expect a proportion of patients to be accidently sensitized to A.s. , perhaps in the same proportion as in the general population (about 20 %). These patients would mainly be non-responders to dietary recommendations like those not sensitized to the nematode.
The second possibility is that the clinical outcome of A.s. induced acute or chronic urticaria is dependent on the levels of specific immunoglobulins. We previously showed higher immunoglobulin levels in acute gastro-allergic Anisakiasis compared to sensitized chronic urticaria patients with similar time intervals between 3 and 15 months 26 . Thus we pro-pose a model in which the prognosis of urticaria induced by the parasite A.s. depends on the levels of the different immunoglobulin isotypes. High IgE and IgG4 producers respond with acute urticaria or chronic urticaria with a good response to diet, whereas low IgE and IgG4responders are prone to evolution of CU, which is longer lasting and does not respond to a diet without fish.
With our data we cannot clarify if these different clinical responses are idiotype specific (IgE, IgG or IgG4, IgA), dependent on the live parasite or even on a subsequent frequent contact with proteins of “dead parasite” in parasitized fish or even if the antibodies are only cross-reacting 27,28 .
One fascinating possibility would be that IgE or other idiotype antibodies against A.s. could be directed against thermostable antigens of “dead larvae” in patients frequently exposed to fish 28 . This would be a secondary reaction after an acute parasitism and would explain daily hives not to disappear until the patient has no further contact with the responsible antigen.
It is well known that helminth infestations lead to a dramatic induction of the Th2 lymphocyte subset, resulting in stimulation of IgG4 and IgE isotypes 9 . IgG4 antibodies have been reported to be longer-last-ing and a possible role as blocking antibodies has been proposed. Even if allergic manifestations are seldom observed in helminthosis, blocking antibodies or the ratio of IgE to IgG4 have been proposed in determining the expression of allergic symptoms 10,11 . But in a previous study we showed that in gastro-al-lergic Anisakiasis all immunoglobulin subsets (IgE, IgG, IgG4, IgA, IgM) are produced in response to an acute parasitism 13 . Our results roughly give IgG4 a protective role with respect to CU. But it shall not be forgotten that the immune response to this nematode would probably not be necessary in terms of prevention of a chronic infestation as the human being is is not an intermediate or final host in the life cyclus of A.s . Therefore we observed in the present study that independently of the possible predictive nature of specific IgG4, their values have to be interpretated taking into account the levels of total IgE, specific IgE and specific IgG.
In conclusion, our results demonstrate a possible causal association between A. s. and CU, although the mechanisms implicated can up to now only be hypothetical. IgG4 antibodies reflect previous acute parasitism and their absence is associated with a low IgE response and a worser clinical outcome. The described model motivates to further investigate the mechanisms by which microbial agents or parasites are able to induce CU.
Correspondence:
A. DaschnerSección de Alergia Hospital Universitario La Princesa Diego de León, 62 28006 Madrid