Increasing evidences suggest that allergy may reduce the risk of glioma, so it is necessary to perform an up-to-data literature search and investigate this relationship by meta-analysis.
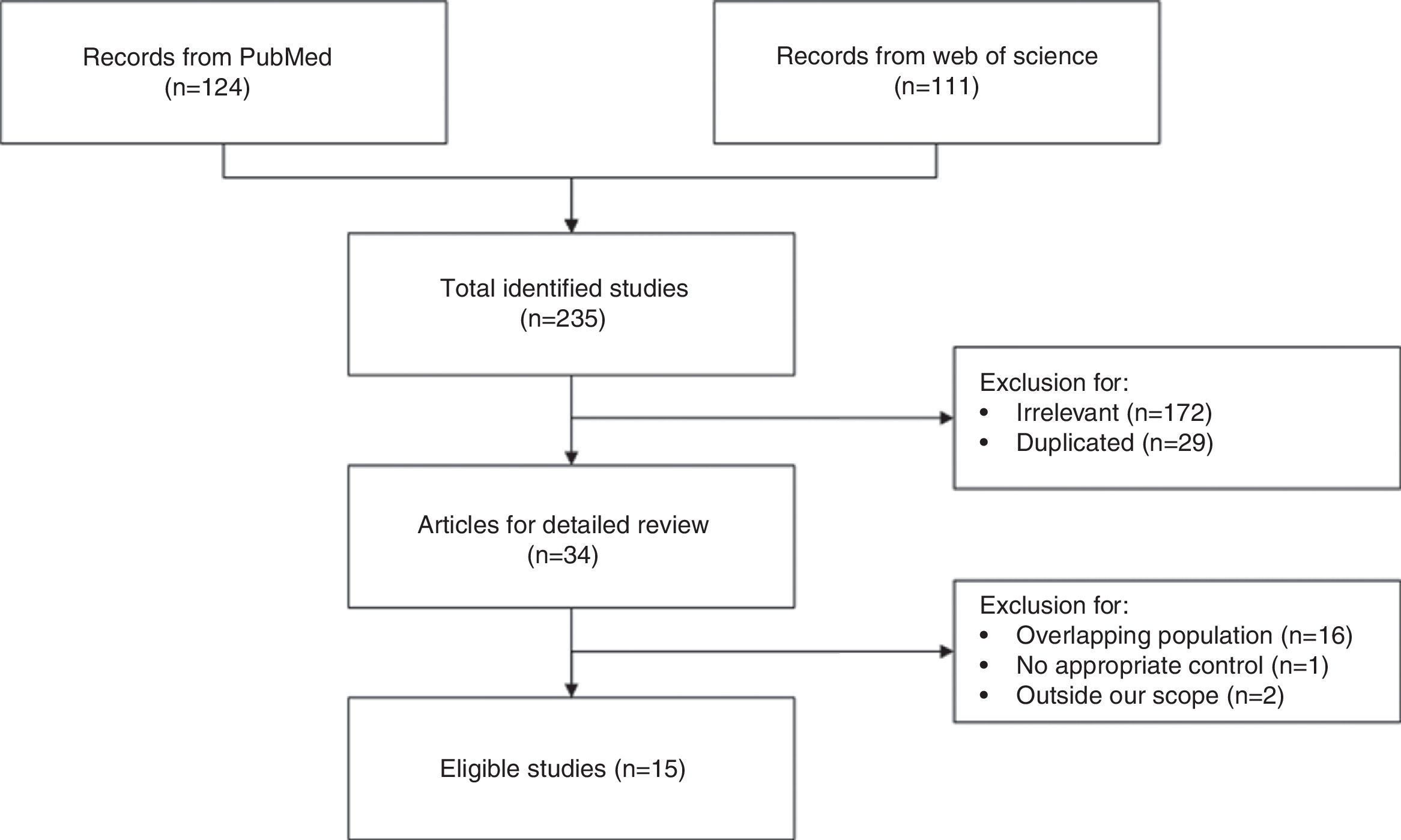
MethodsWe identified the included studies by searching PubMed and Web of Science and excluding irrelevant or ineligible articles. Nineteen studies from 15 articles, including 8435 cases and 118,719 controls, were selected for data extraction and synthesis.
ResultsPooled outcomes showed that there was an inverse association between allergy and risk of glioma (OR=0.64, 95% CI=0.52–0.78, P<0.001). Meanwhile, asthma and eczema would reduce the risk of glioma by 33% and 23% (OR=0.67, 95% CI=0.59–0.75, P<0.001; OR=0.77, 95% CI=0.68–0.86, P<0.001), respectively. Sensitivity analyses confirmed the stability of these findings. Besides, no publication biases were detected regarding all the investigations.
ConclusionsOverall or specific allergy is protective against glioma. More prospective cohort studies or molecular laboratory experiments are warranted to elucidate the causation and key mechanism.
Glioma is the most common primary brain tumour and remains incurable. The most aggressive form, glioblastoma multiforme (GBM), has an overall 15–17-month survival time.1 Like other neoplasms, glioma develops and progresses as a result of genetic and molecular alterations.2 Besides, immune system may have an impact on the pathogenesis of this cancer,3 although the traditional assumption has been that immune responses in the central nervous system (CNS) are limited. Allergic diseases are on the rise, with increasing interest in their long-term health effects. These disorders, including asthma and eczema, have been consistently related to lower risk of glioma in epidemiological studies.4–8 The latest meta-analysis conducted in 2013 concluded that allergic conditions were reversely related to the risk of glioma.9 Apart from this meta-analysis, recently an unprecedented study with 14 recruitment sites across five countries, the Glioma International Case-Control Study (GICC), has reported that respiratory allergies, asthma and eczema have been significantly protective against glioma.10
Due to inclusion of overlapping populations, update of the cohorts that have been published previously8,11,12 and the emergence of a new study,6 we conducted an up-to-date literature search, excluded duplicated populations and pooled outcomes of the eligible trails, which aimed to confirm the already existing relationship between allergy and glioma.
Methods and materialsLiterature search and selectionA comprehensive literature search was carried out in PubMed and Web of Science databases to identify the relevant articles published from Jan 1979 to Oct 2016. The search terms were “glioma”, “brain tumour”, “allergy”, “asthma” and “eczema”. No special restrictions were imposed regarding type of publication. The irrelevant records were initially discarded according to titles and abstracts. The remaining articles were examined carefully to check if they met the inclusion criteria as follows: (1) case–control or cohort study with special interest on the relationship between allergy and glioma; (2) provided odds ratios (ORs) and 95% confidence intervals (95% CIs) or had enough data to calculate them; (3) reported the newest data of the recruited populations; (4) reported the largest sample of the populations during the same period. The eligible studies were ultimately included in the data synthesis.
Data extractionThe detailed information and data were recorded by two authors independently. The extracted items were: study name, study period, numbers of cases and controls, ORs, 95% CIs and adjusted confounders. ORs were calculated if they were not provided by the original articles. Disagreements on data extraction and calculation were solved after consultation.
Data synthesisThe heterogeneity within studies was assessed by Q-statistic.13 It was measured by I2 value, which indicated the percent of the total variance across studies due to heterogeneity rather by chance. Heterogeneity was categorised into high, medium or low when I2≥50%, 50%>I2≥25% or 25%>I2, respectively.14 If no heterogeneity existed, Mantel–Haenszel's method in fixed effect was used to pool outcomes. Otherwise they were accumulated by Dersimonian and Laird method in random effect model.15 Publication bias was evaluated by the symmetry of funnel plot and by Egger's linear regression test statistically.13 Sensitivity analysis was performed by omitting each study and checking whether the pooled result changed significantly. All statistical analyses were conducted by Stata 9.0 (Stata Crop LP, College station, TX, USA). All P values were two-sided and identified as significant if less than 0.05.
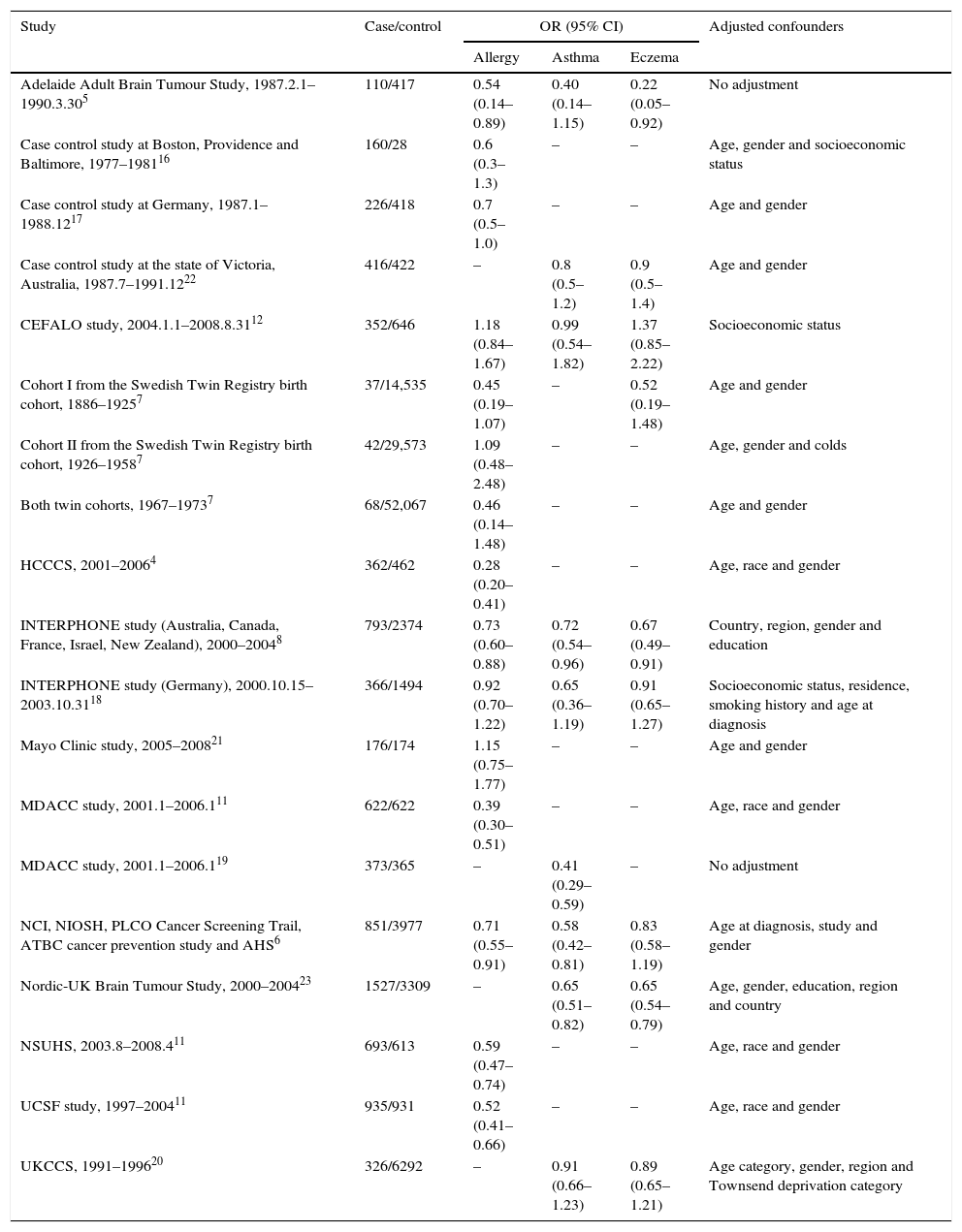
ResultsCharacteristics of the eligible studiesAs shown in Fig. 1, nineteen studies from 15 articles,4–8,11,12,16–23 including 8435 cases and 118,719 controls, were finally included in this meta-analysis. Fifteen of the included studies4–8,11,12,16–18,21 reported the ORs of the associations between allergy and glioma. Meanwhile some studies paid attention to specific allergic diseases, such as asthma and eczema. Nine studies5,6,8,12,18–20,22,23 investigated the relationships between asthma and glioma and a further nine studies5–8,12,18,20,22,23 focused on eczema and glioma. The majority of the ORs extracted from the studies were adjusted for age and gender, except for two studies with no adjustment5,19 (Table 1).
Characteristics of the included studies.
| Study | Case/control | OR (95% CI) | Adjusted confounders | ||
|---|---|---|---|---|---|
| Allergy | Asthma | Eczema | |||
| Adelaide Adult Brain Tumour Study, 1987.2.1–1990.3.305 | 110/417 | 0.54 (0.14–0.89) | 0.40 (0.14–1.15) | 0.22 (0.05–0.92) | No adjustment |
| Case control study at Boston, Providence and Baltimore, 1977–198116 | 160/28 | 0.6 (0.3–1.3) | – | – | Age, gender and socioeconomic status |
| Case control study at Germany, 1987.1–1988.1217 | 226/418 | 0.7 (0.5–1.0) | – | – | Age and gender |
| Case control study at the state of Victoria, Australia, 1987.7–1991.1222 | 416/422 | – | 0.8 (0.5–1.2) | 0.9 (0.5–1.4) | Age and gender |
| CEFALO study, 2004.1.1–2008.8.3112 | 352/646 | 1.18 (0.84–1.67) | 0.99 (0.54–1.82) | 1.37 (0.85–2.22) | Socioeconomic status |
| Cohort I from the Swedish Twin Registry birth cohort, 1886–19257 | 37/14,535 | 0.45 (0.19–1.07) | – | 0.52 (0.19–1.48) | Age and gender |
| Cohort II from the Swedish Twin Registry birth cohort, 1926–19587 | 42/29,573 | 1.09 (0.48–2.48) | – | – | Age, gender and colds |
| Both twin cohorts, 1967–19737 | 68/52,067 | 0.46 (0.14–1.48) | – | – | Age and gender |
| HCCCS, 2001–20064 | 362/462 | 0.28 (0.20–0.41) | – | – | Age, race and gender |
| INTERPHONE study (Australia, Canada, France, Israel, New Zealand), 2000–20048 | 793/2374 | 0.73 (0.60–0.88) | 0.72 (0.54–0.96) | 0.67 (0.49–0.91) | Country, region, gender and education |
| INTERPHONE study (Germany), 2000.10.15–2003.10.3118 | 366/1494 | 0.92 (0.70–1.22) | 0.65 (0.36–1.19) | 0.91 (0.65–1.27) | Socioeconomic status, residence, smoking history and age at diagnosis |
| Mayo Clinic study, 2005–200821 | 176/174 | 1.15 (0.75–1.77) | – | – | Age and gender |
| MDACC study, 2001.1–2006.111 | 622/622 | 0.39 (0.30–0.51) | – | – | Age, race and gender |
| MDACC study, 2001.1–2006.119 | 373/365 | – | 0.41 (0.29–0.59) | – | No adjustment |
| NCI, NIOSH, PLCO Cancer Screening Trail, ATBC cancer prevention study and AHS6 | 851/3977 | 0.71 (0.55–0.91) | 0.58 (0.42–0.81) | 0.83 (0.58–1.19) | Age at diagnosis, study and gender |
| Nordic-UK Brain Tumour Study, 2000–200423 | 1527/3309 | – | 0.65 (0.51–0.82) | 0.65 (0.54–0.79) | Age, gender, education, region and country |
| NSUHS, 2003.8–2008.411 | 693/613 | 0.59 (0.47–0.74) | – | – | Age, race and gender |
| UCSF study, 1997–200411 | 935/931 | 0.52 (0.41–0.66) | – | – | Age, race and gender |
| UKCCS, 1991–199620 | 326/6292 | – | 0.91 (0.66–1.23) | 0.89 (0.65–1.21) | Age category, gender, region and Townsend deprivation category |
Abbreviations: UCSF, The University of California, San Francisco; NSUHS, Duke and North Shore University Health System; MDACC, MD Anderson Cancer Center; HCCCS, Harris County Case Control Study; NCI, National Cancer Institute; NIOSH, National Institute for Occupational Safety and Health; PLCO, the Prostate, Lung, Colorectal and Ovarian; ATBC, Alpha-Tocopherol, Beta-Carotene; AHS, Agricultural Health Study; UKCCS, UK Childhood Cancer Study.
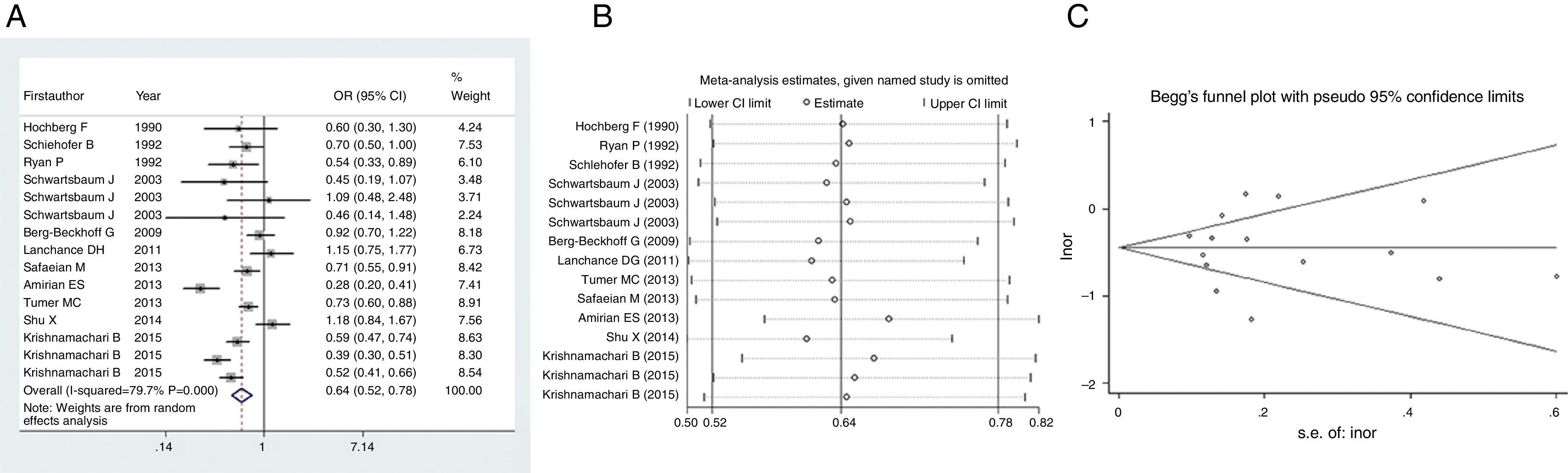
Firstly, we pooled the results describing relationship between allergy and risk of glioma. Since heterogeneity within studies was found (I2=79.7%, P<0.001), we employed random effect to estimate the effect. As shown in Fig. 2A, there was an inverse association between allergy and risk of glioma (OR=0.64, 95% CI=0.52–0.78, P<0.001), suggesting that history of allergy would reduce the risk of glioma by 36%. Sensitivity analysis indicated that this result was stable since no study omission would significantly affected the estimated relationship (Fig. 2B). No publication bias was detected by funnel plot (Fig. 2C) or Egger's test (t=0.03, P=0.978).
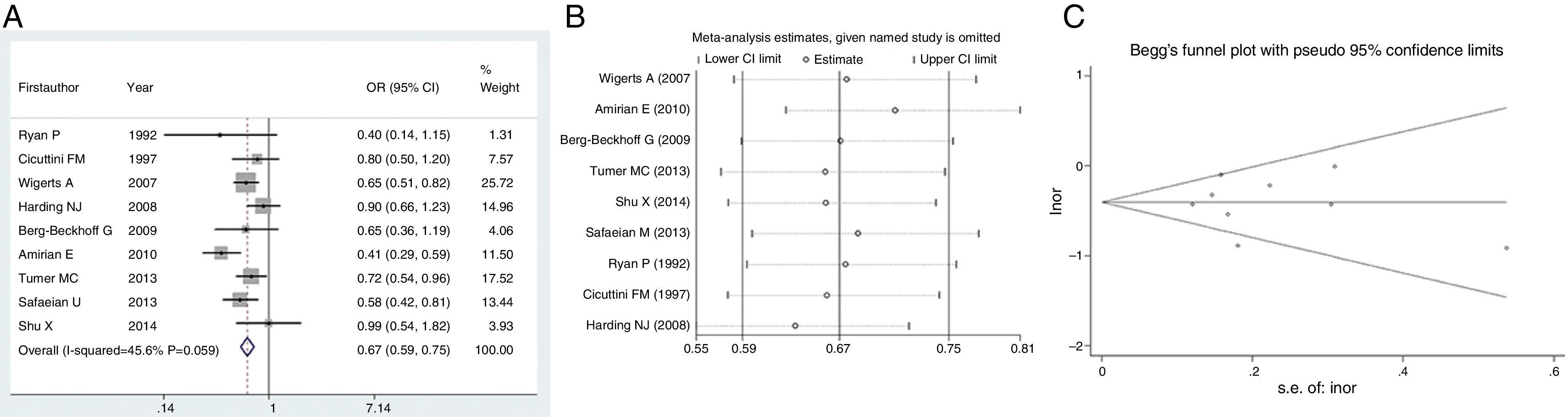
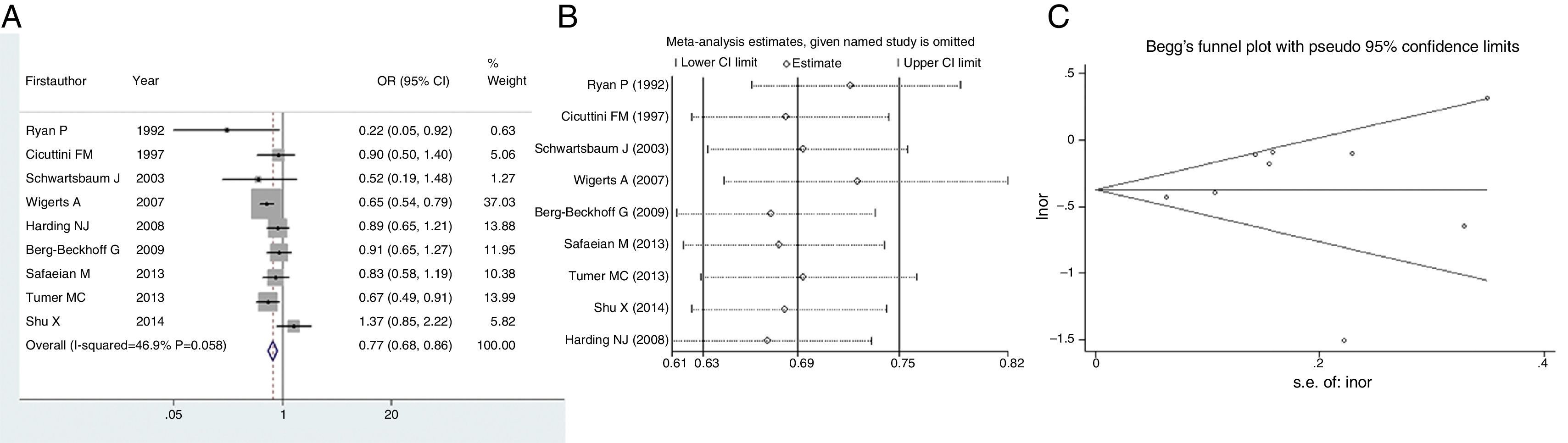
We next evaluated the protection of glioma conferred by asthma or eczema. In terms of asthma, the pooled result indicated that this immune disease would reduce the risk of glioma by 33% (OR=0.67, 95% CI=0.59–0.75, P<0.001) (Fig. 3A). Likewise, eczema would reduce the risk of glioma by 23% (OR=0.77, 95% CI=0.68–0.86, P<0.001) (Fig. 4A). Sensitivity analyses confirmed that both results were stable (Figs. 3B and 4B), and no publication biases were detected for the both estimations (Figs. 3C and 4C).
Gliomas are tumours that arise from glial or precursor cells.24 The broad category glioma represents approximately 28% of all brain tumours and 80% of malignant tumours. These tumours account for about 53% of brain tumours in children ages 0–14 years.24 The aetiology of glioma has for many years not been very well understood. It has been indicated that genetic variants,19 radiation exposure,25 virus infection26 and unhealthy lifestyles27,28 are risk factors for this cancer. On the other hand, increasingly studies have consistently reported a protective effect of allergy on glioma.4–8
In this meta-analysis, which was based on the newest data from novel or existing cohorts, we discovered that allergy would significantly reduce the risk of glioma by 36%. This result was stable and reliable according to the sensitivity analysis and publication bias detection. Consistently, a meta-analysis collecting literature from Jan 1979 to Oct 2013 discovered that allergic condition was inversely associated with the risk of glioma (OR=0.78, 95% CI=0.73–0.83).9 Another meta-analysis published much earlier also found a protective role of overall allergic conditions on glioma (OR=0.60, 95% CI=0.52–0.69).29 Taking these three analyses together, allergy would notably reduce the risk of glioma by 22–40%.
In the meantime, some studies also reported the relationship between a specific allergic condition, such as asthma or eczema, and risk of glioma. Therefore, we evaluated the protective effect of asthma or eczema as well. Similarly, pooled results indicated asthma or eczema would reduce the risk of glioma by 33% or 23%, respectively. A recent meta-analysis has identified studies from 1979 to 2015 and has found that history of eczema to be associated with decreased risk of glioma (OR=0.69, 95% CI=0.61–0.78).30 The meta-analysis we mentioned earlier29 also investigated the specific allergic diseases, reporting individual ORs as 0.70 (95% CI=0.62–0.79), 0.69 (95% CI=0.62–0.78) and 0.78 (95% CI=0.70–0.87) for asthma, eczema and hay fever, respectively. According to the latest information provided by the GICC study with 14 recruitment cites, having respiratory allergies, asthma and eczema were all significantly protective against glioma.10 Due to these consistent discoveries, in is almost a confirmation that overall or specific allergy is inversely associated with glioma.
Allergic condition is mediated by type 2 immune responses, as it depends on IgE antibodies and Th2 cells. Some studies have provided information and suggested that the protective effect of allergy on glioma has been trustworthy in the context of molecular mechanism. Firstly, the amount of IgE is remarkably increased in allergy. The potent capability of IgE to kill tumour cells via antibody-dependent cellular cytotoxicity has been demonstrated in various mouse models.31 Moreover, clinical studies have initiated to test the efficacy of mouse-human chimeric IgE antibodies against solid tumours.32 In line with that evidence, a systematic review has shown a decreased risk of glioma related with elevated level of total IgE (RR=0.74, 95% CI=0.62–0.88).33 Secondly, it is hypothesised that a highly active immune system leads to an enhanced tumour immune surveillance through recognising and killing tumour cells. On the one hand, allergic individuals have exhibited lower frequencies of Treg cells,34 while these cells have been suggested to have a prominent role in gliomagenesis.35 On the other hand, allergy provokes the emergence of a Th2 cell response.36 The anti-tumour functions mediated by Th2 immunity have been well-established. The advantage of Th2 cells is that they can amplify the innate anti-tumour immune response by IL-4 production and eosinophils recruitment, resulting in tumour suppression and clearance.37
In addition, this meta-analysis should be interpreted with some cautions. Firstly, we intended to provide the latest and comprehensive information by including all available cohorts with the most recent outcomes or with the largest samples. Unfortunately, we found that many articles summarised the findings from different recruitment sites, rather than reporting the individual values.6,20,23,38 Considering that a given population was followed up, included in studies over years and reported as a part of the outcome from a large multicentre study, it was difficult to extract the latest data of this cohort. Therefore, we believe that apart from the overall findings, the detailed descriptions of individual outcomes from individual recruitment sites are necessary. Secondly, a causal conclusion cannot be drawn since case–control studies cannot provide the timeline of aberrant immune response to glioma occurrence. As we know, the pathogenesis of allergic diseases entails an ineffective tolerogenic immune response to allergens and Treg cells are essential to control allergy by immunosuppressive functions.36 Of note, Treg cells have been present among glioma patients and associated with a more aggressive clinical course.35 Macrophages and microglia within glioma microenvironment produce CCL2, a chemokine that is crucial for recruiting Treg cells, thus facilitating tumour accumulation.39 In this scenario, there is also a biological plausibility that glioma is able to provide a suppressive milieu where allergy is blocked. Some studies have aimed to clarify the temporal order of allergy prior to tumour by obtaining information about allergy many years preceding glioma diagnosis.7,18,38,40 Inverse associations were both observed when allergy initiated at an early age or when it was present many years before tumour onset,7,18,38,40 therefore reverse causation is unlikely to interpret the present findings.
In conclusion, allergic conditions are associated with a remarkable reduced risk of glioma. More prospective cohort studies are warranted to confirm the causation between allergy and decreased risk of glioma. In addition, studies investigating the molecular mechanism which explains and manipulates this protective effect are encouraged.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestsNone.