Storage mites of the genus Acarus can be responsible for allergic sensitisation in domestic environments. Acarus gracilis is a frequent species in some geographical regions of the Iberian Peninsula. Since the allergenicity of this mite has not been described before, the objectives of this study were to characterise it immunologically, and to compare it with the closely related and more extensively studied species Acarus siro.
MethodsExtracts from A. gracilis and A. siro cultures were characterised by Lowry, 1D and 2D-SDS and IEF. Zymogram, and determination of different enzymatic activities were performed. Skin prick solution of A. gracilis was tested in consecutive patients attending the Hospital of Mérida (Extremadura, Spain). Serum samples from eight individuals with positive skin prick test were collected. IgE determination, immunoblot and immunoblot-inhibition studies were performed.
ResultsExtracts of both species showed a very similar protein and allergenic profile. Allergens at 14 and 17kDa were clearly recognised in both extracts by serum samples. Immunoblot-inhibition studies demonstrated that both extracts were totally inhibited by the opposite one. Enzymatic activity was similar in both cases with the most important differences being in kallikrein, serine protease and collagenase activities.
ConclusionThe storage mite A. gracilis has a similar protein and allergen profile to A. siro and can induce allergic sensitisation. Due to the higher prevalence of this species respect to A. siro in some regions, more studies are needed to determine the clinical significance of sensitisation to this storage mite species.
Storage mites of the genus Acarus (Astigmata: Acaridae) are well known as important pests of stored food products.1 The most important of them belong to the Acarus siro complex, composed of three species with different environmental preferences,2 with high similarities among them but genetically distinct.3 The most common and cosmopolitan of this group is A. siro L., present in a wide variety of outdoor and indoor habitats. This species has been related with occupational allergies including bakers,4 cheese producers5 or cereal workers,6 and has also been associated with indoor respiratory allergy, mainly in rural areas.7 Different allergens of A. siro have been identified until now, including a protein of 15kDa, termed Aca s 13, homologous to the fatty acid-binding protein (FABPs) allergens. Previous studies have shown that Aca s 13 was recognised by 23% of patients sensitised to this mite.8,9 The other species currently included in the A. siro complex; A. farris (Oudemans) and A. immobilis Griffiths, have been found mainly in outdoor environment and have a lesser allergological importance.
Besides these species, another member of the genus, Acarus gracilis Hughes, has been described as an outdoor and indoor species.10 Records are not numerous, but it has been found in stored products and domestic dust, in different countries such as Greece,11 Germany,12 England,13 Portugal,14,15 Spain,16 and Australia,17 although its capacity to induce allergic symptoms has never been identified nor investigated. Molecular studies of A. gracilis showed a high similarity with the species from A. siro complex, though lesser than between species of the complex, suggesting a basal position of A. gracilis in relation to them.3 In a recent survey of domestic mites in Spanish mattress's dust, A. gracilis was much more prevalent and abundant than A. siro. Specially striking was the presence of A. gracilis in inland Western regions of Spain, such as specific areas in Castilla y León, Andalucía, Galicia, and especially Extremadura, where its presence was detected in more than 29% of the investigated samples, whereas A. siro was only found in 1%.18
Since the allergenicity of this mite has not been described before, the objectives of this study were to characterise an A. gracilis extract, and to compare it with the closely related and more extensively studied species A. siro.
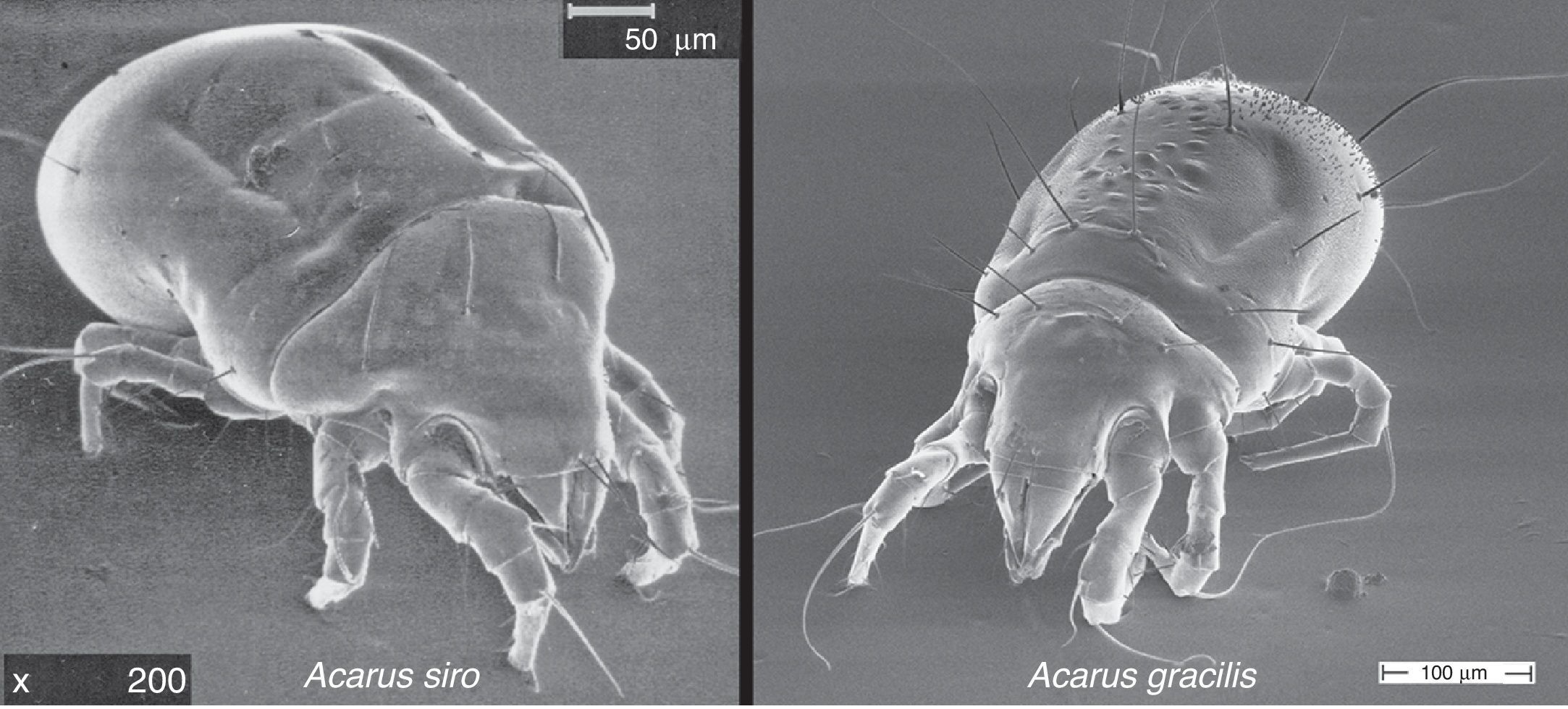
Material and methodsRaw material and extractSpecimens of A. gracilis (CSL, Sand Hutton, UK), and A. siro (Laboratorios LETI, Madrid, Spain), identified by light and scanning electron microscopy (Fig. 1), were cultured in yeast in controlled conditions of temperature and humidity (25°C: 75% RH) during one month. Whole cultures were frozen to kill the mites and sieved in order to separate mite bodies from the culture medium. Fractions with mite bodies had a richness (number of mite particles/total particle number×100) higher than 80%.
Extracts from A. gracilis and A. siro were obtained after extraction of the body fractions (1:20wt/vol) in 0.01M PBS (pH 7.4) for 10h. Thereafter, the extracts were centrifuged at 16,000×g at 4°C for 30min and the supernatants collected, filtered, dialysed against bidistilled water in dialysis membranes of 3.5kDa (CelluSep Membrane, Seguin, TX, USA), sterile filtered, frozen and freeze-dried. Skin prick solutions were manufactured with A. gracilis extract at a concentration of 2mg/ml.
Patient selectionPatients with positive skin prick test to A. gracilis (a positive skin test reaction was defined as a wheal size greater than 3mm in diameter), were selected from new patients attending the Allergy Service of the Hospital of Mérida (Extremadura, Spain), reporting respiratory and/or cutaneous allergic symptoms. Choice of the area was done because of the high prevalence of A. gracilis in the region.18 Oral informed consent for skin testing and eventual blood sampling was obtained from patients during the allergy consult. Serum samples from a total of eight individuals were obtained, and a pool was prepared by mixing equal amount of sera with specific IgE to A. gracilis.
Extract characterisationExtracts from A. siro and A. gracilis were characterised and studied in parallel. The protein content of the extracts was measured by the Lowry–Biuret method (Sigma, St. Louis, MO, USA). Results were expressed in micrograms of protein per milligram of freeze-dried material.
Protein profile of both extracts was studied by 1-D SDS–PAGE, bidimensional gels (2-D) and isoelectric focusing (IEF electrophoresis). While 1-D was performed with crude extract, IEF and (2D) were performed with a purified protein solution, obtained after a concentration and purification of the native extract (at 2mg/ml) with ammonium sulphate. Finally, samples were centrifuged, the pellet collected and then reconstituted in ultra-purified water. Concentrated extract was washed using ReadyPrep 2-D Cleanup Kit (Bio-Rad), following the manufacturer's instructions.
For 1-D electrophoresis samples were run on SDS–PAGE gels. One hundred micrograms of freeze-dried material of both extracts were prepared. Samples were diluted in sample buffer, denatured at 100°C for 10min and centrifuged for 1min at 16,000×g. After electrophoresis, the gel was stained with Coomassie® Blue R250 (Bio-Rad, Hercules, CA, USA).
IEF gel (6.25% T; 3.3% C) was prepared in a range of pH from 3 to 10 (Bio-Lyte® ampholines, Bio-Rad). A total of 16μg of each extract was loaded. Gels were revealed with Coomassie® Blue.
For 2D electrophoresis, extracts were separated according to their isoelectric point in ReadyStrip IPG Strips (Bio-Rad) in a pH range between 3 and 10, using Protean IEF Cell (Bio-Rad). After the first dimension, the strip was equilibrated with the ReadyPrep 2-D Kit buffers (Bio-Rad) and proteins were separated in the second dimension according to their molecular weight. Gels were revealed with SYPRO® Ruby stain (Bio-Rad) and revealed under UV light.
The immunological profile of individuals was carried out using the individual sera (only A. gracilis). Allergenic profile of both extracts was compared with the pool of sera. The gel was electrophoretically transferred to P-immobilon membrane (PVDF, Millipore, Bedford, MA, USA) and dried at room temperature for 4h. The membrane was incubated overnight with the sera. Specific IgE binding was detected with peroxidase-conjugated monoclonal anti-human IgE (Ingenasa, Madrid, Spain). Immunoblots were revealed with peroxide buffer/luminol enhancer (Immun-Star™ WesternC™ kit, Bio-Rad) by chemiluminescence. Images were captured and edited with Quantity One® software, v4.5.1 (Bio-Rad) and ImageQuant™ TL, v 2005 (GE Healthcare).
Enzymatic activityEnzymatic activity was determined in both mite extracts. A complete battery of analyses was performed in order to fully characterise the extracts and to investigate the activity of the allergens, following previously described methods.19
ZymogramA. gracilis and A. siro extracts were run on SDS–PAGE gels with 2.67%C, 15%T polyacrylamide containing 0.1% gelatine. After running, gels were incubated with 2.5 Triton X-100 (Bio-Rad) during 30min, they were then incubated with a 0.06% L-Cys (Sigma) solution during 90min at 37°C. Finally, gels were stained with Coomassie brilliant blue R-250.
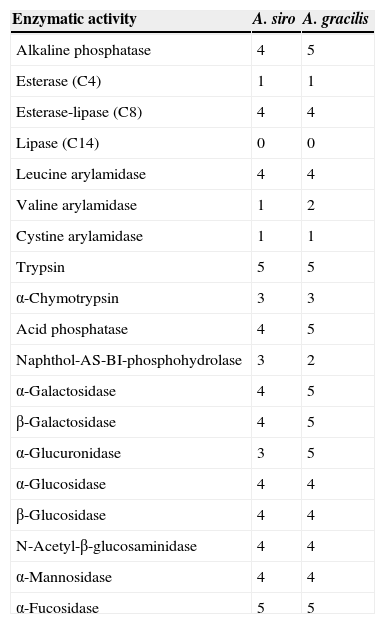
API Zym studiesThe API Zym assay (BioMérieux, France) was used to determine the enzymatic activity of 19 different enzymes. Values ranged from 0 to 5, with 0 being absence of activity and 5 the maximum activity. Results were interpreted according to the manufacturer's instructions.
Individual enzymatic activityCysteine protease activity was determined using the L-1460 peptide (Pyr-Phe-Leu-p-nitroanilide; Bachem, Bubendorf, Switzerland) as substrate and papain (Sigma) as standard (results expressed in μg of papain/mg of freeze-dried material).
Serine protease activity was evaluated using D-Ile-Pro-Arg-p-nitroanilide dihydrochloride (Sigma) as a substrate and trypsin from bovine pancreas (Thermo Scientific, Kanagawa, Japan) as standard. Results were expressed in trypsin equivalents per milligram of protein.
Kallikrein protease activity was determined in mite extracts using the L-1885 peptide (H-D-Val-Leu-Arg-pNa acetate; Bachem AG) as the substrate and trypsin as the standard. Results were expressed in μg of trypsin/milligram of protein.
Collagenase activity was determined using azocoll (Sigma) according to previously reported methods20 and manufacturer information. Trypsin (Sigma) was used as a standard.
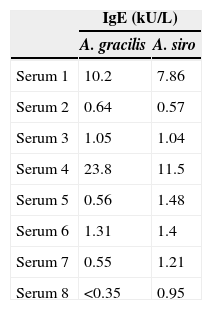
IgE determinationThe patients’ sera were screened for specific IgE (sIgE) to both Acarus species using UniCAP 100® system (Phadia, Uppsala, Sweden). sIgE to A. siro was measured by commercial ImmunoCAP™, whereas sIgE to A. gracilis was quantified using StreptavidinCAP™ loaded with a biotinylated (Roche Diagnostics, Indianapolis, IN, USA) A. gracilis extract, following previously described methods.21
Immunoblot inhibitionCross-reactivity experiments were carried out using A. gracilis and A. siro extracts in solid phase. A total amount of 100μg of each extract was transferred to a nitrocellulose membrane following the procedure previously described.
The serum pool was incubated beforehand for 2h with A. siro extract (in case of A. gracilis in solid phase) or A. gracilis (in case of A. siro in solid phase) (500μg/ml), and then added to the membranes and incubated overnight. As a secondary antibody, a monoclonal mouse anti-human IgE (Ingenasa, Madrid, Spain) was used. The immunoblot was developed by chemiluminescence.
Statistical analysisMann–Whitney rank test was used for the comparison of IgE values between species. SigmaStat 3.5 (Point Richmond, CA, USA) software was used for the analysis.
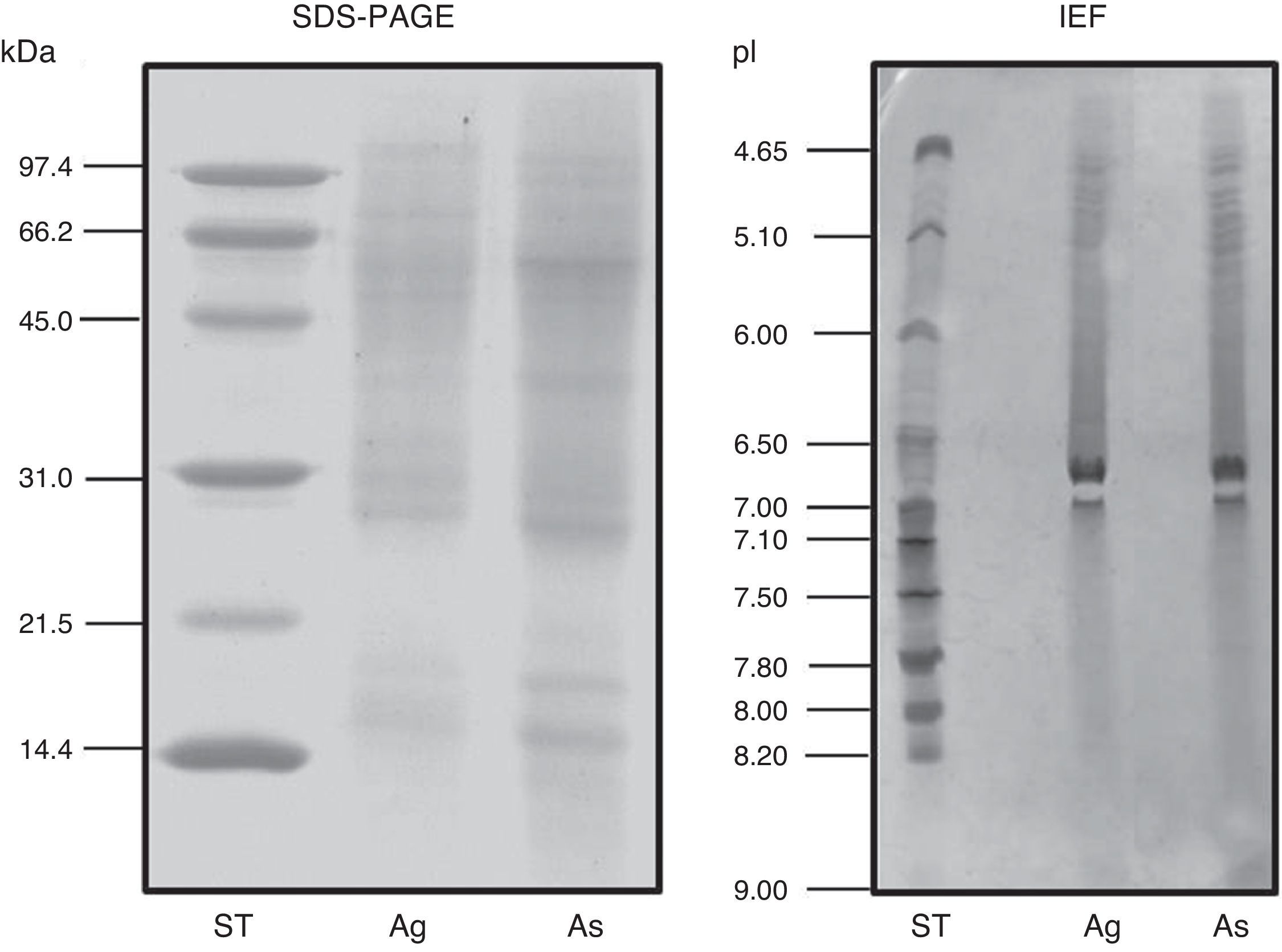
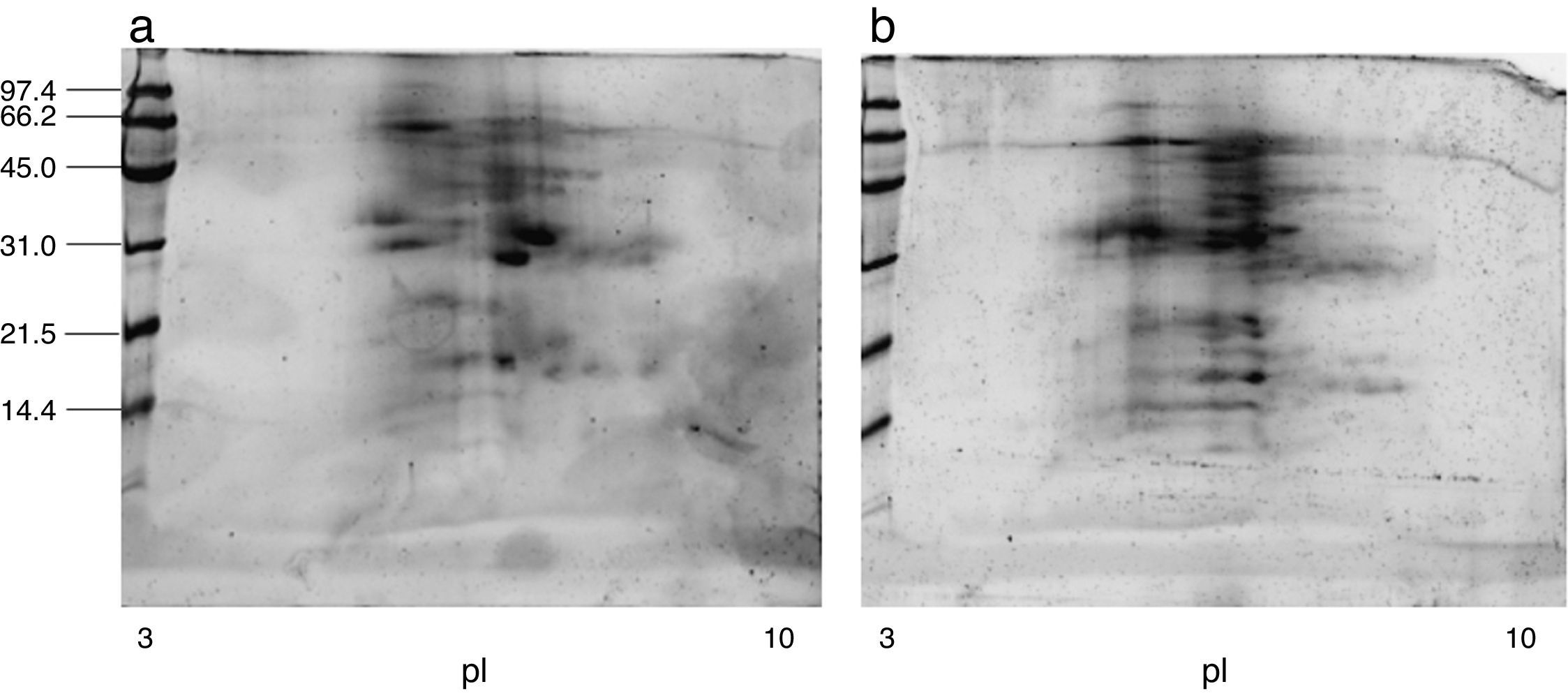
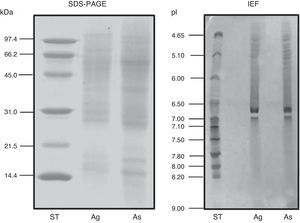
ResultsExtract preparation and protein profileThe protein content of the A. siro extract was 520μg protein/mg and 381μg protein/mg in A. gracilis extract. The protein profile, analysed by SDS and matching between both extracts evidenced the presence of numerous common proteins with the same molecular weight, in a range between 10 and 100kDa. The most relevant bands were visualised at 15–17, 25–30 and 55–62kDa (Fig. 2). The same fact was observed by IEF (Fig. 2) and 2-D SDS (Fig. 3).
Gelatinolytic gels (zymogram) showed that both extracts have similar enzymatic activity mainly detected in a band of approximately 27kDa.
About individual enzymatic activity, A. siro showed higher levels than A. gracilis of kallikrein (18.27 vs. 5.91μg papain/mg prot) and serine protease (4.27 vs. 1.85μg trypsin/mg prot), whereas collagenase activity was higher in A. gracilis (7.09 vs. 4.98μg trypsin/mg prot). Cysteine protease activity (9.28 vs. 9.03μg trypsin/mg prot) and most of the 19 enzyme assays in the API Zym studies demonstrated similar enzymatic activity (Table 1).
Enzymatic activity results obtained with API Zym (values ranging from 0 to 5, with 0 being no and 5 maximal activity).
| Enzymatic activity | A. siro | A. gracilis |
|---|---|---|
| Alkaline phosphatase | 4 | 5 |
| Esterase (C4) | 1 | 1 |
| Esterase-lipase (C8) | 4 | 4 |
| Lipase (C14) | 0 | 0 |
| Leucine arylamidase | 4 | 4 |
| Valine arylamidase | 1 | 2 |
| Cystine arylamidase | 1 | 1 |
| Trypsin | 5 | 5 |
| α-Chymotrypsin | 3 | 3 |
| Acid phosphatase | 4 | 5 |
| Naphthol-AS-BI-phosphohydrolase | 3 | 2 |
| α-Galactosidase | 4 | 5 |
| β-Galactosidase | 4 | 5 |
| α-Glucuronidase | 3 | 5 |
| α-Glucosidase | 4 | 4 |
| β-Glucosidase | 4 | 4 |
| N-Acetyl-β-glucosaminidase | 4 | 4 |
| α-Mannosidase | 4 | 4 |
| α-Fucosidase | 5 | 5 |
The results of specific IgE obtained by the UniCAP 100® system for the eight sera are represented in Table 2. The average specific IgE concentration for A. gracilis (4.8±8.4kUA/L) was higher than for A. siro (3.2±4.1kUA/L), but without statistically significant differences (P=0.382).
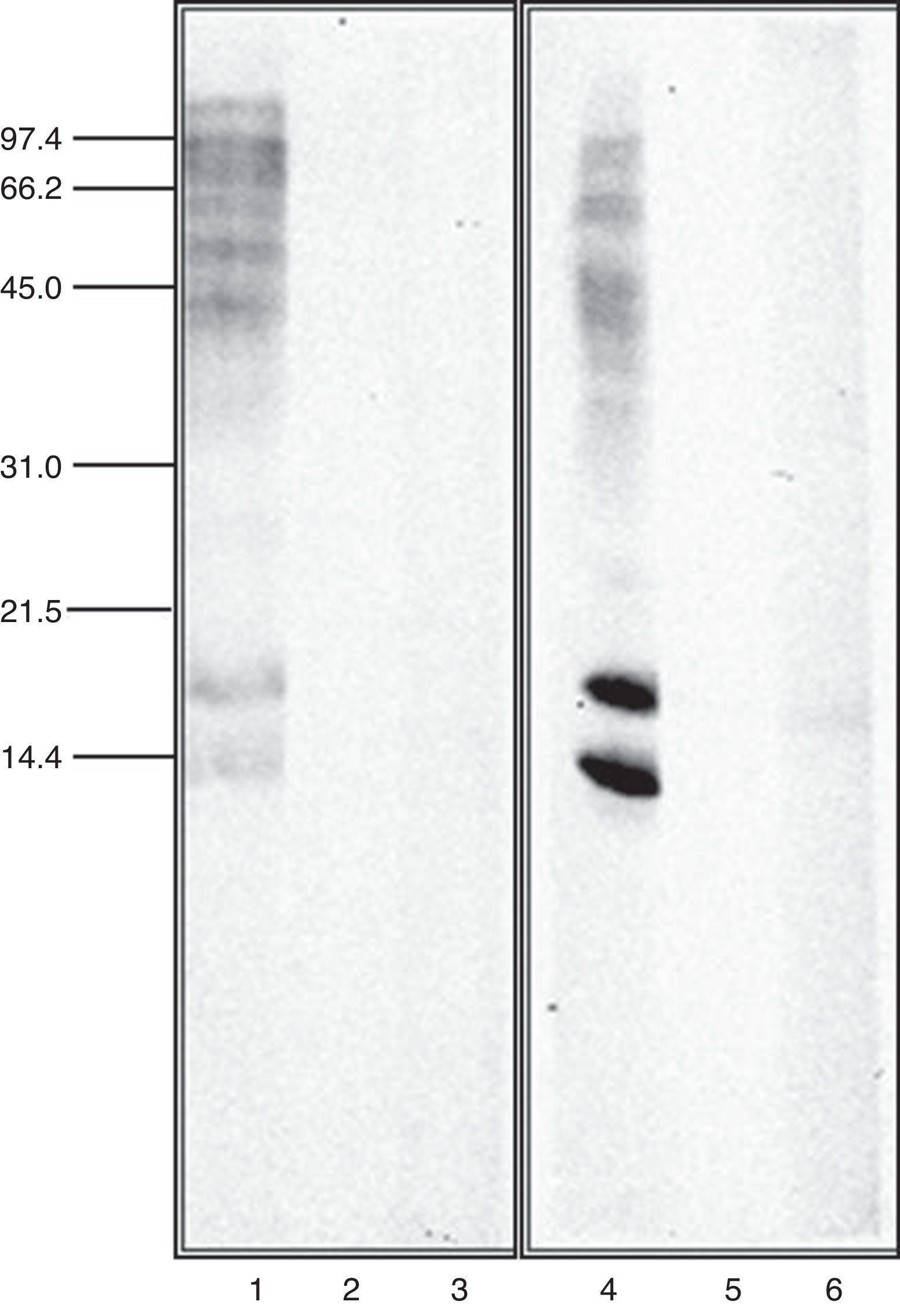
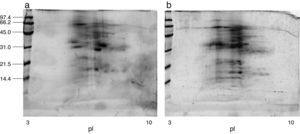
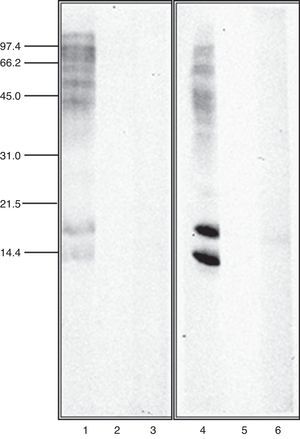
Immunoblot inhibition studiesThe allergenic profile of both species was similar (Fig. 4: Lanes 1 and 4), although the recognition of the 14 and 17kDa bands was more intensive in A. siro. The immunoblot inhibition studies showed that both extracts were totally inhibited by the opposite one (Fig. 4: Lanes 3 and 6).
DiscussionFor a correct diagnosis and treatment of mite allergy it is essential to identify the domestic mite fauna to which patients are exposed. That was the objective of a major survey of indoor mite exposure in Spain18 which showed the high relative presence of not well known species from an allergenic point of view. That was the case of the storage A. gracilis, which was found in 3.7% of mattress dust samples, being considerably higher in western-inland areas, such us Extremadura (29% of dust samples). In comparison, the most known Acarus species, A. siro, was present in only 0.6% of the dust samples in Spain, and 1.1% in Extremadura.
Although the prevalence of sensitisation to A. gracilis has not been evaluated in this study, and it is not possible to assure the real percentage of sensitised population, it seems that the allergological importance of this species should be investigated in detail. These noteworthy differences lead us to investigate the allergological importance of A. gracilis. According to our results, the allergenic capacity of A. gracilis has been confirmed. In parallel, the results confirm that this species is totally related with those observed in A. siro suggesting that the sensitisation rates would be similar. Morphologically, A. gracilis resembles A. siro but it can be easily distinguished by the wrinkled cuticle and the presence of minute papillae on the posterior region of the body (Fig. 1).
Until now, nothing was known about the allergenic relevance of A. gracilis or its capacity to induce allergic symptoms. The objectives of this study were to characterise immunochemically the A. gracilis extract and to investigate its capacity to induce specific IgE. A. gracilis and A. siro are highly related from a taxonomical point of view.3 Seven allergens of A. siro have been listed to date (www.allergome.org), although three of them (Aca s 1, Aca s 3 and Aca s 8) are included as in silico allergens, and there is no reported information about sensitisation to three other allergens (Aca s 2, Aca s 7 and Aca s 10). The only allergen listed in the IUIS database is Aca s 13, a fatty acid-binding protein of 15kDa recognised by 23% of A. siro RAST-positive patients.8 No information has been published about A. gracilis allergens.
The protein profile of the two analysed species was very similar and non-significant differences were observed. In both cases bands of approximately 15–17 (which could correspond with Aca s 13), 25–30 and 55–62kDa, were clearly visualised. Other proteins with high molecular weight were also identified. 2-D analysis showed a similar profile in both species, with several isoforms detected in different bands. A. siro showed more clearly visualised bands in low molecular weight allergens, probably related with the higher protein content. The existence of proteins with high molecular weight was also confirmed in both species. IEF studies did not show important differences and confirmed the results obtained with 2-D. These results confirmed the similarities between both species.
It is well documented that the enzymatic activity of several mite allergens, and protease activity in particular, is considered one of the main reasons for the capacity of these proteins to induce allergic sensitisation.22 In the present study we considered that the enzymatic activity of the extracts of A. gracilis, respect to A. siro, could be related with the capacity to induce allergic sensitisation of A. gracilis. We demonstrated high similarities between them and with other storage mites23 that feed on the protein-rich endosperm of grains, with a number of proteinases that are not inhibited by the grain's components, as with cysteine protease.24 However, differences in collagenase activity which could be used to digest collagenous substrates in mammal skin cells,25 suggest that A. gracilis could have different ecological requirements than A. siro, being more adapted to the indoor environment.
The allergenic profile of the extracts, investigated by immunoblot analysis confirmed a similar pattern of recognition in both extracts. Allergens at 15–17kDa were clearly recognised, probably related with the group 13 of allergens described in A. siro. Other allergens with higher molecular weight were also identified in both extracts.
Cross-reactivity between mites has been widely documented, mediated by common or related allergens. Broadly speaking, cross-reactivity among species of different groups of storage mites, and between storage mites and house dust mites can be considered limited.26 However it is considered important between taxonomically related storage mite species27 Cross-reactivity between the two species of Acarus included in the study was investigated in detail by immunoblot inhibition. The experiments confirmed a total inhibition between A. siro and A. gracilis, demonstrating a high degree of cross-reactivity. This idea was suggested before, since polyclonal antibodies against protein fractions from A. siro reacted with A. gracilis, whereas no cross-reaction was observed with other Acaridae mites.28
In summary, we have demonstrated the allergenic capacity of A. gracilis, the similar protein profile and the high degree of cross-reactivity with A. siro. More studies, in particular, nasal provocation tests are needed to determine the clinical significance of sensitisation to this storage mite species.
FundingThe study was financially supported by Laboratorios LETI (Madrid, Spain).
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflict of interestThe authors have no conflict of interest to declare.