Corticosteroids are potent anti-inflammatories and immunomodulators used in the treatment of various inflammatory and allergic reactions that can cause delayed and immediate hypersensitivity, often misdiagnosed because of atypical clinical presentation.1 There are four main groups (A, B, C and D with subgroups D1 and D2) defined by their chemical properties and depending on their molecular structure. Skin metabolism plays an important role in the allergenicity of corticosteroids and also influences the profile of cross-reactions.2–3
A 31-year-old-Caucasian female, lawyer, was assisted in the emergency room, after an exuberant swelling of her eyelid caused by an insect bite. She was treated initially with intravenous hydrocortisone and after discharge, with deflazacort (6mg per day), clarithromycin and topical hydrocortisone. The patient returned to the emergency room 24h later, for a widespread rash showing some individualised erythematous-pink plaques with circinate limits and pustules, interpreted as an urticarial reaction or as an acute exanthematous pustulosis.
She denied personal or family history of atopy, but reported an episode of acute dermatitis of the face caused by topical anti-acne cream, ten years before. Hypersensitivity to neomycin, tixocortol pivalate, Kathon CG, thimerosal, nickel, cobalt and benzoyl peroxide had been demonstrated in patch test performed that time.
The following immunoallergology tests were carried out: prick tests and intradermal tests with deflazacort 6mg, hydrocortisone and clarithromycin in increasing dilutions. In addition, patch tests were performed with the basic series adopted by (PCDG), a corticosteroid series (including: dipropionate, betamethasone valerate, dexamethasone, triamcinolone, clobetasol, prednicarbat, mometasone, hydrocortisone and tixocortol pivalate), her own drugs and the other drugs already tested in immunoallergology.
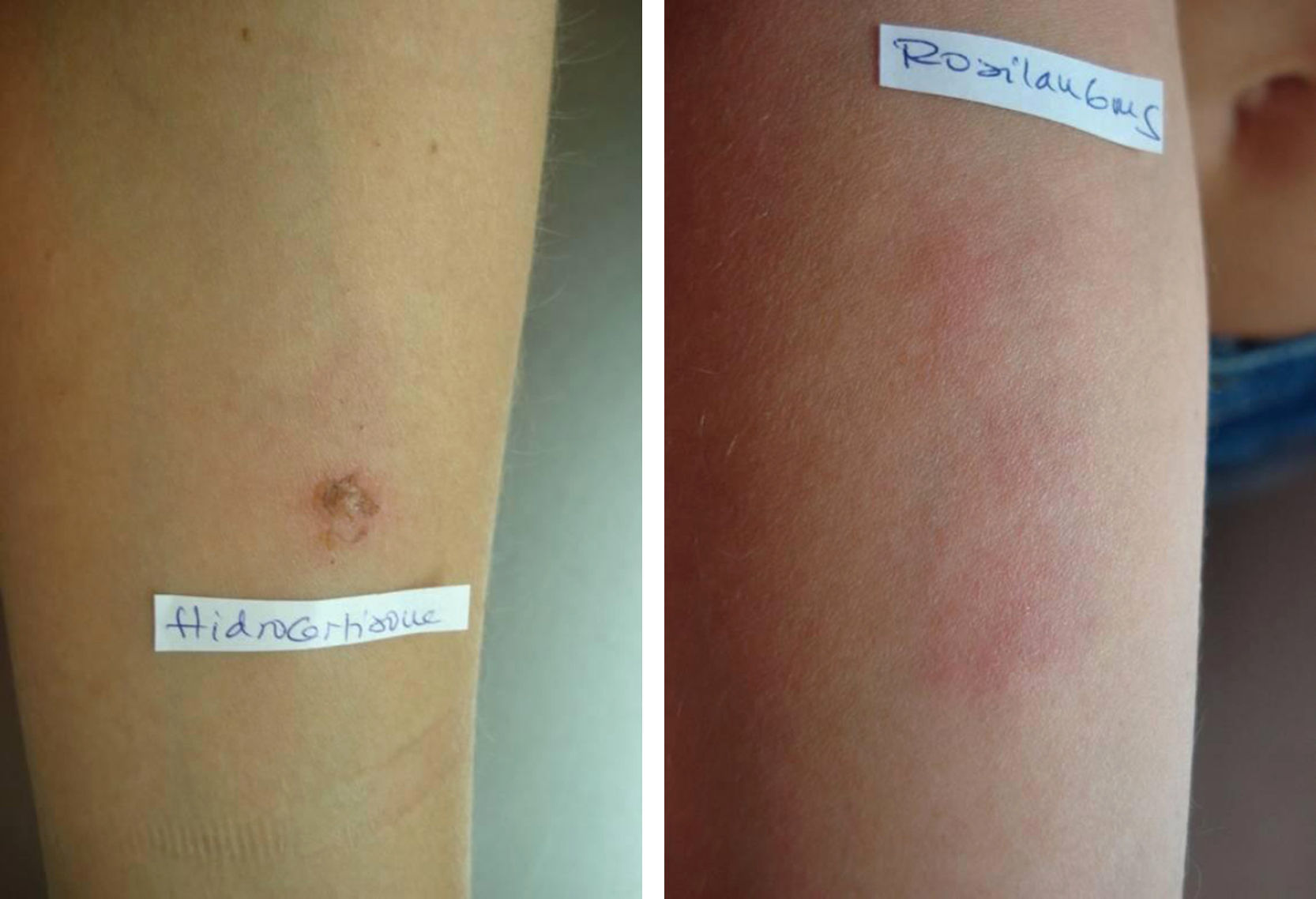
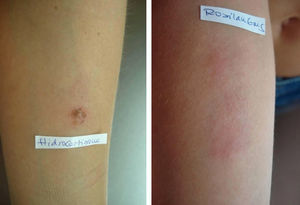
Prick tests and intradermal tests: read for 20min – negative. (immunoallergology). The first positive results were noted (by the patient) after 12h: hydrocortisone and deflazacort 6mg (Fig. 1, Table 1).
Hypersensitivity was shown to tixocortol pivalate (a Group A substance), as well as to hydrocortisone (Group A) and deflazacort. They have a similar structure to methylprednisolone (which also belongs to Group A) (Table 2).
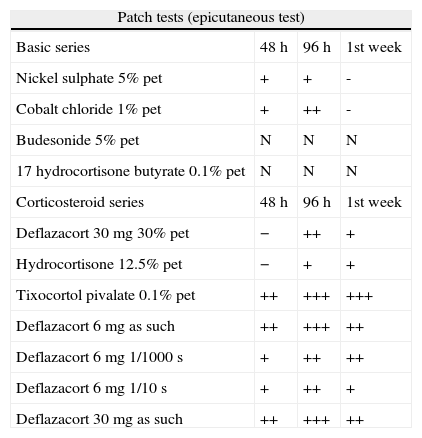
Evidence from reading patch tests with the basic series adopted by PCDG, corticosteroids and the patient's own drugs in increasing dilutions.
| Patch tests (epicutaneous test) | |||
| Basic series | 48h | 96h | 1st week |
| Nickel sulphate 5% pet | + | + | - |
| Cobalt chloride 1% pet | + | ++ | - |
| Budesonide 5% pet | N | N | N |
| 17 hydrocortisone butyrate 0.1% pet | N | N | N |
| Corticosteroid series | 48h | 96h | 1st week |
| Deflazacort 30mg 30% pet | − | ++ | + |
| Hydrocortisone 12.5% pet | − | + | + |
| Tixocortol pivalate 0.1% pet | ++ | +++ | +++ |
| Deflazacort 6mg as such | ++ | +++ | ++ |
| Deflazacort 6mg 1/1000s | + | ++ | ++ |
| Deflazacort 6mg 1/10s | + | ++ | + |
| Deflazacort 30mg as such | ++ | +++ | ++ |
As an option to find alternative therapies, we proceeded to prick, intradermal tests and patch test with methylprednisolone, dexamethasone sodium phosphate, betamethasone and prednisolone in increasing dilutions: as such, 1/10, 1/100, 1/1000 in saline.
Positive readings were obtained 12h after the intradermal tests were started (prednisolone and methylprednisolone), while the patch tests were negative (Table 3).
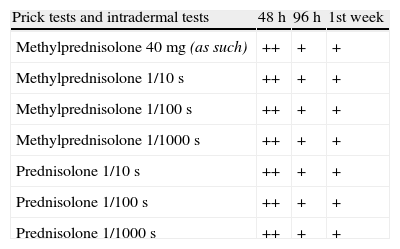
Evidence from reading prick tests and intradermal tests with corticosteroids in increasing dilutions given by systemic administration.
| Prick tests and intradermal tests | 48h | 96h | 1st week |
| Methylprednisolone 40mg (as such) | ++ | + | + |
| Methylprednisolone 1/10s | ++ | + | + |
| Methylprednisolone 1/100s | ++ | + | + |
| Methylprednisolone 1/1000s | ++ | + | + |
| Prednisolone 1/10s | ++ | + | + |
| Prednisolone 1/100s | ++ | + | + |
| Prednisolone 1/1000s | ++ | + | + |
The prevalence of allergy to corticosteroids is highly variable: contact allergic reactions occur in 0.2 to 5% of patients who undergo patch tests while the immediate hypersensitivity is rare, representing 0.1–0.3%.4
Allergic reactions may also arise from systemic administration, although the prevalence is not known.4 The published cases of reaction to deflazacort are rare.5–6
Deflazacort is a derivative component of prednisolone, given by oral intake and widely used because of its minimal adverse effects. It has a similar structure to methylprednisolone, with a connection with oxazoline in C17.5 Some authors believe that it does not cross-react with other corticosteroids.7
Corticosteroids are classified into four groups, depending on their molecular structure and chemical properties. Cross-reactions occur mainly between corticosteroids within the same group, while the cross-reactions between groups are not frequent.7
Tixocortol pivalate is involved in almost 90% of type IV reactions.4
In the case of this patient, hypersensitivity to tixocortol pivalate (marker of Group A), found in tests carried out 10 years before, irrelevant at that time, can be considered premonitory of the later reaction to deflazacort. Intradermal tests were positive at 48h and 96h readings for hydrocortisone, which also belongs to group A, as well as for deflazacort 6mg in different dilutions. The epicutaneous tests confirmed hypersensitivity to tixocortol pivalate in vaseline, to hydrocortisone (also in vaseline) and increasing dilutions in saline, as well as to deflazacort 6mg. Although the preparation of active ingredients in ethanol is being indicated,4 it was not practically feasible to carry out those tests.
Intradermal tests become positive earlier than the patch tests. The results from both tests were in agreement most of the time,4 as occurs in this case.
It can be assumed that the clinical evolution was a response comparable to the one reproduced during testing (delayed reaction).
The results obtained with the prick and intradermal tests with commercial preparations of systemic administration (methylprednisolone, dexamethasone, sodium phosphate, betamethasone and prednisolone) confirmed hypersensitivity to methylprednisolone and prednisolone, both in Group A. These tests may be extremely helpful, finding alternative drugs that may play a vital role for the patients, which in this case was dexamethasone.
The metabolism of corticosteroids in the skin can also lead to cross-reactions between classes, if the molecules behave as haptens before and after bio-metabolisation.2–5 Deflazacort and methylprednisolone (Group A) share structural similarity, which may explain a cross or concomitant reaction.
Soria et al.4 advocate that patch tests with the active ingredients diluted in ethanol are the preferred diagnostic method for delayed hypersensitivity to corticosteroids. Intradermal tests with delayed readings can detect additional cases. However, they should not be performed routinely because of the risk of atrophy.
Ethical disclosuresProtection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Patients’ data protectionThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.