Cannabis is the illicit drug most widely used by young people in high-income countries. Allergy symptoms have only occasionally been reported as one of the adverse health effects of cannabis use.
ObjectivesTo study IgE-mediated response to cannabis in drug users, atopic patients, and healthy controls.
MethodsAsthmatic patients sensitised to pollen, and all patients sensitised to tobacco, tomato and latex, considered as cross-reacting allergens, were selected from a data base of 21,582 patients. Drug users attending a drug-rehabilitation clinic were also included. Controls were 200 non-atopic blood donors. Specific IgE determination, prick tests and specific challenge with cannabis extracts were performed in patients and controls.
ResultsOverall, 340 patients, mean age 26.9±10.7 years, were included. Males (61.4%) were the most sensitised to cannabis (p<0.001). All cannabis-sensitised patients were alcohol users. Eighteen (72%) of the patients allergic to tomato were sensitised to cannabis, but a positive specific challenge to cannabis was highest in patients sensitised to tobacco (13/21, 61.9%), (p<0.001). Pollen allergy was not a risk factor for cannabis sensitisation. Prick tests and IgE for cannabis had a good sensitivity (92 and 88.1%, respectively) and specificity (87.1 and 96%) for cannabis sensitisation.
ConclusionsCannabis may be an important allergen in young people. Patients previously sensitised to tobacco or tomato are at risk. Cannabis prick tests and IgE were useful in detecting sensitisation.
It is estimated that, in 2008, more than 500,000 people received medical treatment for drug abuse in the European Union and there were almost 70,000 deaths due to overdose.1 Symptoms often included shock, beginning with respiratory failure and skin rash. Cannabis use was the highest in the USA, Australia and New Zealand, followed by Europe, especially in young people.2
The prevalence of allergic diseases has increased progressively, with prospective studies suggesting that, between 2010 and 2020, 40%-50% of people might be affected, with rhinitis and asthma showing the greatest rising trend, and anaphylaxis being the most severe type.
The suggested risk factors for allergies include genetic predisposition, better hygiene, smoking and climate change, among others, but none explain such a large increase in so few years. Possible sensitisation to drugs has not been widely considered, as drug reactions are generally attributed to toxic causes and allergic hypersensitivity to immunological causes, with the two seen as mutually exclusive.
Our working hypothesis was that there may be allergic hypersensitivity to drugs in risk populations (atopic patients and drug abusers), as both groups have underlying immune deficiencies. Cannabis, which is of vegetable origin, may also sensitise people allergic to plants in the same manner as vegetal allergens.
If cannabis sensitisation could be detected by allergy testing, this might result in diagnostic and therapeutic advances with social, legal, and health repercussions. Adverse drug responses may not be solely toxic. Some drugs, such as penicillin or poisons derived from hymenopters,2 are allergens for which severe hypersensitivity responses have been demonstrated. Drugs might possess vegetable allergens similar to those of pollens and plants and could provoke an immune response in predisposed people,3–8 which could be related to toxic drug reactions, meaning the response is really a toxic-immunological mechanism. Young, productive people are affected by both types of disorder and there could be a nexus of union between them.
ObjectivesThe objectives of our study were to evaluate allergic hypersensitivity mediated by IgE to Cannabis in drug abusers and allergic patients with a possibility of cross-reactivity with cannabis (latex, tomato and tobacco)8–14 and the diagnostic yield (sensitivity, specificity and predictive values) of routine tests (skin prick, specific IgE, specific challenge) in determining cannabis allergy as determined by bronchial response.
Patients and methodsWe carried out an observational case-control study using the CONSORT guidelines, an evidence-based set of recommendations for randomised controlled trials.10 Asthma patients of both sexes allergic to pollen and residing in Valladolid city or province who had lived in the same house from birth (ensuring that all had been exposed to similar levels of pollens, pollution and other environmental factors) and fulfilled similar clinical criteria of asthma severity were randomly selected from the registry of 21,582 patients attended in the last 20 years by the Allergy Clinic, Rio Hortega University Hospital of Valladolid, Spain.
The data on atmosphere quality were kindly provided by the excellence group on Physical Atmosphere of Valladolid University, GOA-UVA, (http://goa.uva.es). All the patients were studied by the same doctor, using standardised extracts and the same diagnostic methods.
Pollen sensitivity was defined as: a) one or more positive skin tests for pollen; b) CAP (IgE) positive > 0.35 IU/mL for pollen; or c) positive specific challenge. Asthma due to gramineae pollens was defined by prick test, specific IgE and spirometry. Lolium perenne, the most important allergen in our region, was chosen as a measurable parameter in the results of pollen tests. The reports on the pollen levels of different vegetable species in our area (kindly sent monthly to our Allergy Section from the Health Protection Service of Directorate of Public Health of our Community, www.salud.jcyl.es), have never detected cannabis pollen in our atmosphere.
Patients sensitised to tomato, tobacco and latex, allergens possibly implicated in cross-reactivity,8–14 and which are contained in the same data base, were also included. These types of sensitisation are infrequent, and therefore all patients attended over the last 20 years were included in the study. Twenty-five patients sensitised to tomato, 25 sensitised to tobacco, and 18 sensitised to latex were recruited.
A group of drug-dependent patients from the Castile and Leon Association for the Aid of Drug Abusers (ACLAD) were recruited and the same tests carried out. After written informed consent was obtained, an epidemiological-clinical survey was carried out including the characteristics and origin of dependence, possible adverse reactions (by questioning close friends or relatives), potential involvement of organs and systems, emergency department (ED) care and treatment required.
The control group was comprised of 200 healthy (non-smokers, non-users of cannabis or other illicit drugs and who had never consulted the Allergy Clinic) blood donors (Blood Donation Unit, SACYL).
Cannabis consumption was self-estimated by patients as non-consumption, experimental, occasional, habitual and dependence.
The protocol was approved by Clinical Research Ethics Committees. All participants in the study gave written informed consent.
The following tests were carried out in all patients and controls:
In vivo testsSkin testsSkin tests, including conventional prick tests for licensed allergens using the European group protocol for the diagnosis of drug hypersensitivity,15 included:
Allergen extractsA standard battery of aeroallergens and foods were employed, including pollens (gramineae, trees, weeds and flowers), mites (Dermatophagoides and storage mites), fungi, antigens to animals and common foods (ALK-Abelló, Madrid, Spain).
Cured tobacco extract, tomato and latex at concentrations of 1mg/ml (Bial, Bilbao, Spain) were also included, as was extract of fresh tobacco leaf at a concentration of 1/10 weight/volume, prepared in our laboratory from uncured cured fresh leaves. Histamine 1/100 and physiological saline solution were used as positive and negative controls, respectively. Papule area was measured after 15minutes and traced for posterior measurement by planimetry. A papule ≥19.62mm2 was considered clearly positive, corresponding to a diameter of 5mm. This area was specified as a cut-off point after study of the ROC curves and was designed to exclude false positives due to irritation.
Preparation of cannabis extractsThe extracts used were: Fresh Cannabis sativa cut top leaves cold-milled and defatted with acetone (2x1; 1:5 [weight/volume] for 1 hour at 4°C), and after drying, extracted with 0.1mol/L Tris-HCl pH 7.5, 10mM EDTA (1:5 [weight/volume] for 1 hour at 4°C). After centrifuging at 9000g for 30minutes at 4°C, the supernatant was dialysed against H2O (cut-off point, 3.5 kd) and lyophilised. This extract was used both for bronchial challenge and skin tests.
Diagnostic and pulmonary function testsBaseline diagnostic spirometry was carried out in all participants.
Specific bronchial cannabis challenge was carried using the Chai technique with modifications as previously described.12Cannabis extracts were diluted to 0.005mg/mL, 0.05mg/mL, 0.5mg/mL, 1mg/mL and 5mg/mL. After full stop titration according to Gleich's technique with extract of fresh cannabis leaf, the dilution that provoked a papule of 7mm2 was determined. This dilution was the initial dose used for the bronchial challenge carried out in consenting patients and controls (141 as whole).
In ACLAD patients, direct inhalatory challenge tests were performed after inhalation of a cannabis cigarette, with forced spirometry at 5, 10 and 15minutes. After baseline spirometry, challenge was carried out if FEV1 was > 80%. For cannabis extract challenge, two millilitres of the extract were introduced in the dosimeter and the patient breathed normally for two minutes. Spirometry was carried out and repeated at 5, 10 and 15minutes. If there was no change in FEV1 or if the reduction was > 10%, the next higher concentration was used until a reduction in FEV1 ≥ 20% was achieved, which was considered a positive bronchial challenge. If the reduction in FEV 1 was near 15%, the patient was instructed to inhale the next higher dilution for one minute only. Late responses were monitored with a Mini Wright peak flow meter at 2, 6, 12, and 14hours after the test, and the best measurement of three chosen. Cannabis extract and tobacco challenges were also performed in consenting controls.
In vitro testsSpecific IgESpecific IgE was determined for a battery of aeroallergens and foods: wheat, barley and rye, milk, alpha-lactalbumin, beta-lactoglobulin, casein, egg white and yolk, fish, and vegetables (vegetables, legumes, nuts, fresh fruit) tobacco, latex and tomato, using the ImmunoCAP System (Phadia, Uppsala, Sweden). Levels of IgE > 0.35 kU/L were considered positive. Biotiniled Cannabis extracts were coupled to Streptavidin-ImmunoCAP solid phase (Streptavidin-ImmunoCAP, Phadia AB. Uppsala, Sweden), according to Sander et al. (2005).16 Specific IgE to Cannabis was determined by the ImmunoCAP System using the above mentioned solid-phases.
Statistical analysisThe association between study variables was analysed using Pearson's Chi2 test. When the number of cells with expected levels > 5 was > 20%, they were calculated using Fisher's exact test or the likelihood ratio test.
The Student's t test for independent samples was used to compare means and when the number of groups to compare was greater, the ANOVA test was used. When these were not applicable, the non-parametric Mann-Whitney U test (for two groups) or Kruskal Wallis H test (for more than two groups) were used.
Statistical significance was established as p<0. 05 and 95% confidence intervals (CI) were calculated as necessary.
The statistical analysis was made using the SPSS version 15.0 programme.
ResultsGlobal description of the sampleOne hundred and sixty-eight patients and 200 controls were initially recruited: of these 28 patients were lost to the study and 140 were finally analysed.
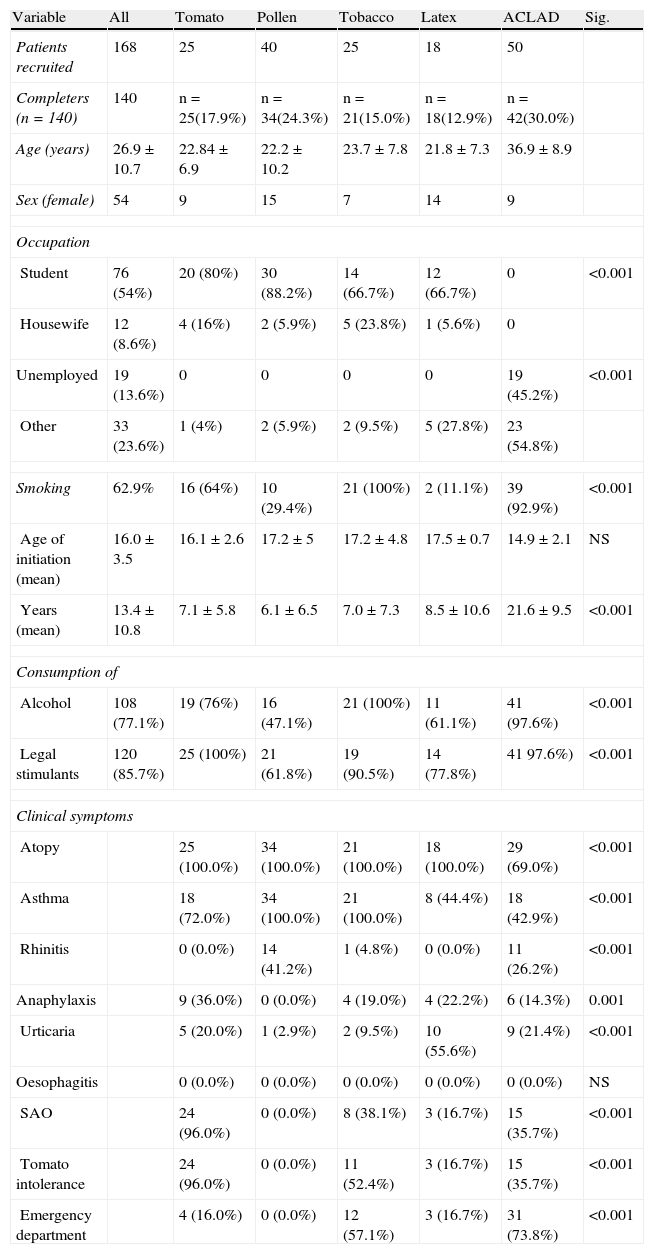
Table 1 shows baseline characteristics of the study groups.
Baseline characteristics of study patients.
| Variable | All | Tomato | Pollen | Tobacco | Latex | ACLAD | Sig. |
| Patients recruited | 168 | 25 | 40 | 25 | 18 | 50 | |
| Completers (n=140) | 140 | n=25(17.9%) | n=34(24.3%) | n=21(15.0%) | n=18(12.9%) | n=42(30.0%) | |
| Age (years) | 26.9±10.7 | 22.84±6.9 | 22.2±10.2 | 23.7±7.8 | 21.8±7.3 | 36.9±8.9 | |
| Sex (female) | 54 | 9 | 15 | 7 | 14 | 9 | |
| Occupation | |||||||
| Student | 76 (54%) | 20 (80%) | 30 (88.2%) | 14 (66.7%) | 12 (66.7%) | 0 | <0.001 |
| Housewife | 12 (8.6%) | 4 (16%) | 2 (5.9%) | 5 (23.8%) | 1 (5.6%) | 0 | |
| Unemployed | 19 (13.6%) | 0 | 0 | 0 | 0 | 19 (45.2%) | <0.001 |
| Other | 33 (23.6%) | 1 (4%) | 2 (5.9%) | 2 (9.5%) | 5 (27.8%) | 23 (54.8%) | |
| Smoking | 62.9% | 16 (64%) | 10 (29.4%) | 21 (100%) | 2 (11.1%) | 39 (92.9%) | <0.001 |
| Age of initiation (mean) | 16.0±3.5 | 16.1±2.6 | 17.2±5 | 17.2±4.8 | 17.5±0.7 | 14.9±2.1 | NS |
| Years (mean) | 13.4±10.8 | 7.1±5.8 | 6.1±6.5 | 7.0±7.3 | 8.5±10.6 | 21.6±9.5 | <0.001 |
| Consumption of | |||||||
| Alcohol | 108 (77.1%) | 19 (76%) | 16 (47.1%) | 21 (100%) | 11 (61.1%) | 41 (97.6%) | <0.001 |
| Legal stimulants | 120 (85.7%) | 25 (100%) | 21 (61.8%) | 19 (90.5%) | 14 (77.8%) | 41 97.6%) | <0.001 |
| Clinical symptoms | |||||||
| Atopy | 25 (100.0%) | 34 (100.0%) | 21 (100.0%) | 18 (100.0%) | 29 (69.0%) | <0.001 | |
| Asthma | 18 (72.0%) | 34 (100.0%) | 21 (100.0%) | 8 (44.4%) | 18 (42.9%) | <0.001 | |
| Rhinitis | 0 (0.0%) | 14 (41.2%) | 1 (4.8%) | 0 (0.0%) | 11 (26.2%) | <0.001 | |
| Anaphylaxis | 9 (36.0%) | 0 (0.0%) | 4 (19.0%) | 4 (22.2%) | 6 (14.3%) | 0.001 | |
| Urticaria | 5 (20.0%) | 1 (2.9%) | 2 (9.5%) | 10 (55.6%) | 9 (21.4%) | <0.001 | |
| Oesophagitis | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | NS | |
| SAO | 24 (96.0%) | 0 (0.0%) | 8 (38.1%) | 3 (16.7%) | 15 (35.7%) | <0.001 | |
| Tomato intolerance | 24 (96.0%) | 0 (0.0%) | 11 (52.4%) | 3 (16.7%) | 15 (35.7%) | <0.001 | |
| Emergency department | 4 (16.0%) | 0 (0.0%) | 12 (57.1%) | 3 (16.7%) | 31 (73.8%) | <0.001 | |
Fifty-five (36.4%) patients had attended the ED, most-commonly for asthma (70.7%), urticaria (19.3%), and anaphylaxis (16.4%). Other frequent allergic symptoms were tomato intolerance (with oral syndrome, 35.7%) and rhinitis. Twenty-six patients (18.6%), suffered from rhinitis. In all cases rhinitis was associated with asthma.
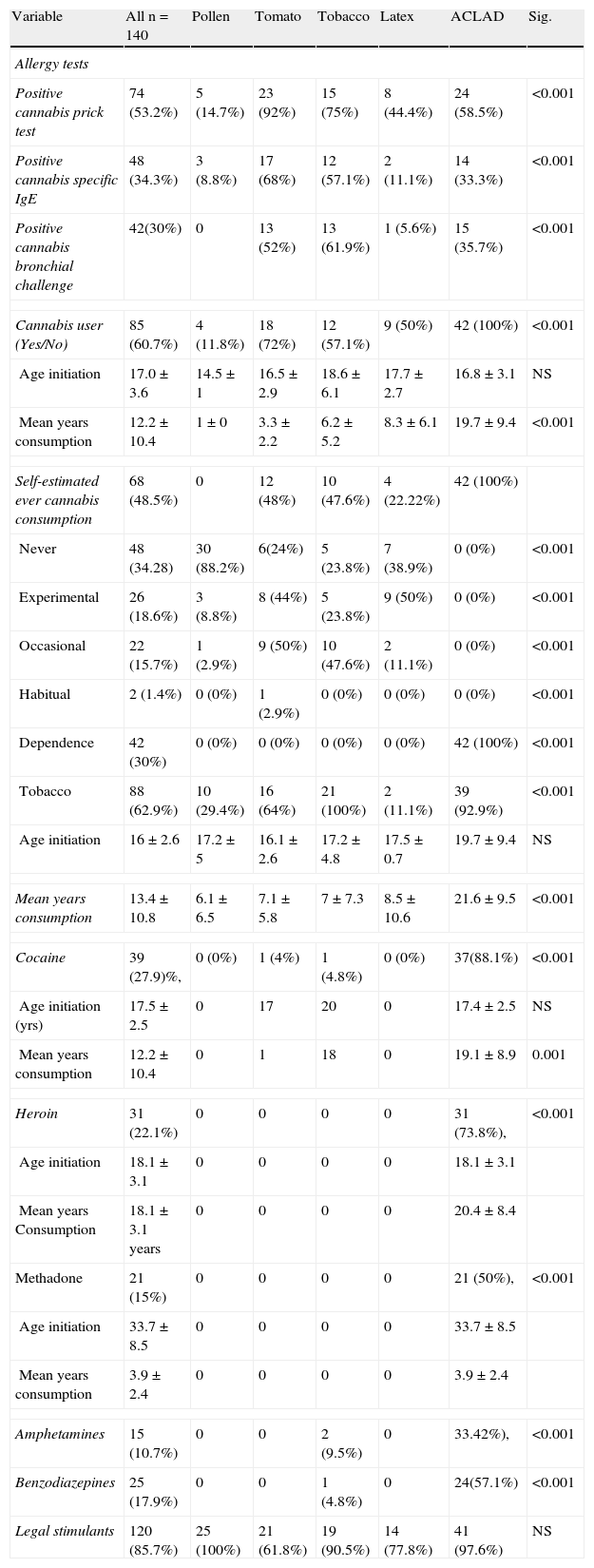
Seventy-four (53.2%) patients had positive skin tests for cannabis, 48 (34.3%) had cannabis-specific IgE and 30% (42 patients) positive bronchial challenge. This would seem to indicate that only 56.7% of positive patients with skin test had positive cannabis challenge, although there may have been positive cannabis prick tests due to cross-reactivity with tomato, latex or tobacco.
The percentage of patients with positive tobacco prick test (37%) was more concordant with positivity of tobacco-specific IgE (44.5%). The same was observed in patients sensitive to tomato (positive prick test 31.2%, positive IgE 34.1%).
Drug consumption and allergy tests (positive cannabis prick test area, positive bronchial challenge and specific IgE) of study patients are shown in Table 2.
Drug consumption and allergic tests in study patients.
| Variable | All n=140 | Pollen | Tomato | Tobacco | Latex | ACLAD | Sig. |
| Allergy tests | |||||||
| Positive cannabis prick test | 74 (53.2%) | 5 (14.7%) | 23 (92%) | 15 (75%) | 8 (44.4%) | 24 (58.5%) | <0.001 |
| Positive cannabis specific IgE | 48 (34.3%) | 3 (8.8%) | 17 (68%) | 12 (57.1%) | 2 (11.1%) | 14 (33.3%) | <0.001 |
| Positive cannabis bronchial challenge | 42(30%) | 0 | 13 (52%) | 13 (61.9%) | 1 (5.6%) | 15 (35.7%) | <0.001 |
| Cannabis user (Yes/No) | 85 (60.7%) | 4 (11.8%) | 18 (72%) | 12 (57.1%) | 9 (50%) | 42 (100%) | <0.001 |
| Age initiation | 17.0±3.6 | 14.5±1 | 16.5±2.9 | 18.6±6.1 | 17.7±2.7 | 16.8±3.1 | NS |
| Mean years consumption | 12.2±10.4 | 1±0 | 3.3±2.2 | 6.2±5.2 | 8.3±6.1 | 19.7±9.4 | <0.001 |
| Self-estimated ever cannabis consumption | 68 (48.5%) | 0 | 12 (48%) | 10 (47.6%) | 4 (22.22%) | 42 (100%) | |
| Never | 48 (34.28) | 30 (88.2%) | 6(24%) | 5 (23.8%) | 7 (38.9%) | 0 (0%) | <0.001 |
| Experimental | 26 (18.6%) | 3 (8.8%) | 8 (44%) | 5 (23.8%) | 9 (50%) | 0 (0%) | <0.001 |
| Occasional | 22 (15.7%) | 1 (2.9%) | 9 (50%) | 10 (47.6%) | 2 (11.1%) | 0 (0%) | <0.001 |
| Habitual | 2 (1.4%) | 0 (0%) | 1 (2.9%) | 0 (0%) | 0 (0%) | 0 (0%) | <0.001 |
| Dependence | 42 (30%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 42 (100%) | <0.001 |
| Tobacco | 88 (62.9%) | 10 (29.4%) | 16 (64%) | 21 (100%) | 2 (11.1%) | 39 (92.9%) | <0.001 |
| Age initiation | 16±2.6 | 17.2±5 | 16.1±2.6 | 17.2±4.8 | 17.5±0.7 | 19.7±9.4 | NS |
| Mean years consumption | 13.4±10.8 | 6.1±6.5 | 7.1±5.8 | 7±7.3 | 8.5±10.6 | 21.6±9.5 | <0.001 |
| Cocaine | 39 (27.9)%, | 0 (0%) | 1 (4%) | 1 (4.8%) | 0 (0%) | 37(88.1%) | <0.001 |
| Age initiation (yrs) | 17.5±2.5 | 0 | 17 | 20 | 0 | 17.4±2.5 | NS |
| Mean years consumption | 12.2±10.4 | 0 | 1 | 18 | 0 | 19.1±8.9 | 0.001 |
| Heroin | 31 (22.1%) | 0 | 0 | 0 | 0 | 31 (73.8%), | <0.001 |
| Age initiation | 18.1±3.1 | 0 | 0 | 0 | 0 | 18.1±3.1 | |
| Mean years Consumption | 18.1±3.1 years | 0 | 0 | 0 | 0 | 20.4±8.4 | |
| Methadone | 21 (15%) | 0 | 0 | 0 | 0 | 21 (50%), | <0.001 |
| Age initiation | 33.7±8.5 | 0 | 0 | 0 | 0 | 33.7±8.5 | |
| Mean years consumption | 3.9±2.4 | 0 | 0 | 0 | 0 | 3.9±2.4 | |
| Amphetamines | 15 (10.7%) | 0 | 0 | 2 (9.5%) | 0 | 33.42%), | <0.001 |
| Benzodiazepines | 25 (17.9%) | 0 | 0 | 1 (4.8%) | 0 | 24(57.1%) | <0.001 |
| Legal stimulants | 120 (85.7%) | 25 (100%) | 21 (61.8%) | 19 (90.5%) | 14 (77.8%) | 41 (97.6%) | NS |
None of the tests performed in the 200 blood donor volunteers were positive for cannabis.
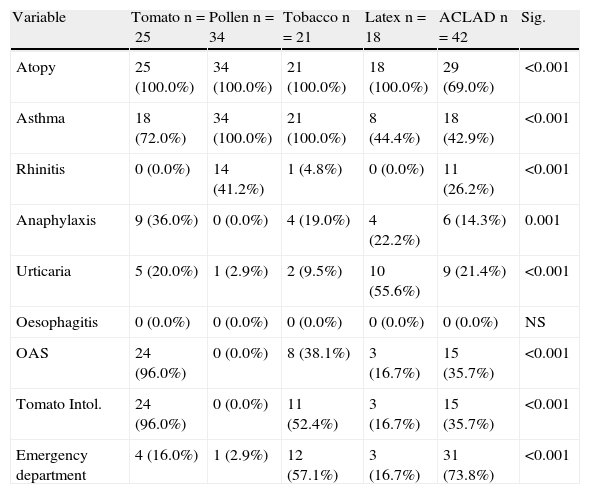
Differences by groupsClinical differences between groups are shown in Table 3.
Differences in clinical variables according to study group.
| Variable | Tomato n=25 | Pollen n=34 | Tobacco n=21 | Latex n=18 | ACLAD n=42 | Sig. |
| Atopy | 25 (100.0%) | 34 (100.0%) | 21 (100.0%) | 18 (100.0%) | 29 (69.0%) | <0.001 |
| Asthma | 18 (72.0%) | 34 (100.0%) | 21 (100.0%) | 8 (44.4%) | 18 (42.9%) | <0.001 |
| Rhinitis | 0 (0.0%) | 14 (41.2%) | 1 (4.8%) | 0 (0.0%) | 11 (26.2%) | <0.001 |
| Anaphylaxis | 9 (36.0%) | 0 (0.0%) | 4 (19.0%) | 4 (22.2%) | 6 (14.3%) | 0.001 |
| Urticaria | 5 (20.0%) | 1 (2.9%) | 2 (9.5%) | 10 (55.6%) | 9 (21.4%) | <0.001 |
| Oesophagitis | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | NS |
| OAS | 24 (96.0%) | 0 (0.0%) | 8 (38.1%) | 3 (16.7%) | 15 (35.7%) | <0.001 |
| Tomato Intol. | 24 (96.0%) | 0 (0.0%) | 11 (52.4%) | 3 (16.7%) | 15 (35.7%) | <0.001 |
| Emergency department | 4 (16.0%) | 1 (2.9%) | 12 (57.1%) | 3 (16.7%) | 31 (73.8%) | <0.001 |
OAS: Oral allergy syndrome. Intol: Intolerance.
Thirty-one patients had attended the ED, six (14.3%) due to anaphylaxis; 18 (42.9%) due to asthma; and nine (21.4%) due to urticaria-angio-oedema. Fifteen (35.7%) also had oral syndrome with raw tomato, but had only excluded tomato from the diet without medical consultation.
Other dependenceOther drugs used included alprazolam (33.33%) and other stimulants in 41 (97.6%) patients. One patient was on insulin.
Twelve patients had HAV, 7 VHB, 13 HCV, and 6 HIV. One patient suffered latex-related nocturnal asthma cured by change of latex mattress.
Of all the asthmatics, 12 presented altered respiratory function tests (obstructive spirometry) at baseline. All patients had a positive bronchial challenge for cannabis.
Patients allergic to tomatoCannabisEight (44%) had used cannabis experimentally only once, nine (50%) occasionally, one habitually, and there was no drug abuser.
In 13 patients (61.9%) with positive IgE and prick test, cannabis challenge was positive and all admitted cannabis use.
Four (16%) had attended the ED after smoking cannabis and drinking alcohol: two with asthma, one with anaphylaxis and one with oral angio-oedema.
No patient tolerated tomato but all tolerated other Solanaceae. Twenty-four (96%) suffered oral syndrome after eating raw tomato.
Patients allergic to gramineae pollenCannabisThree were experimental users and one occasional. There was no habitual or dependent user.
Thirty-one patients tolerated tomato normally. The most frequent disorders were spring asthma (100%) and urticaria (one patient). None had anaphylaxis or angio-oedema or had attended the ED.
Patients allergic to tobaccoCannabisSeven patients were experimental and five were occasional users, with no habitual or dependent user.
Nine (45%) tolerated tomato without oral discomfort and 11 (52%) were intolerant, of whom eight had oral allergy syndrome. Nine (52.85%) were positive for both tests.
The 12 patients sensitised to cannabis had attended the ED, four due to anaphylaxis (after a party at which cannabis, tobacco and alcohol were consumed) and eight due to asthma. All were occasional consumers.
The main disorders were asthma in 21 patients, urticaria in two (9.5%) and anaphylaxis four (19%).
Patients allergic to latexCannabisSeven were experimental users and none was a dependent user.
Three patients were tomato intolerant and three (16.7%) had attended the ED (all users of cannabis, after one night of partying) one due to asthma and two due to anaphylaxis.
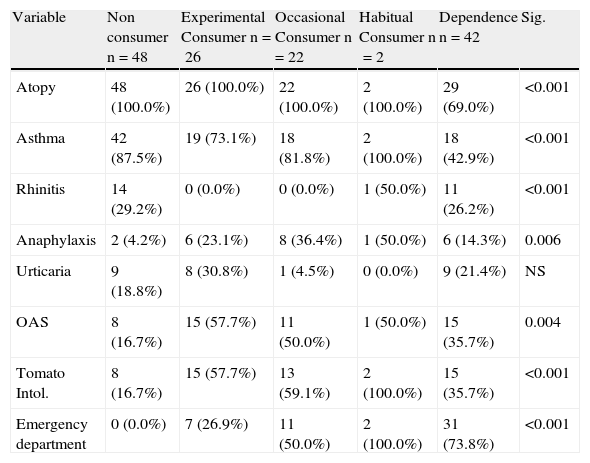
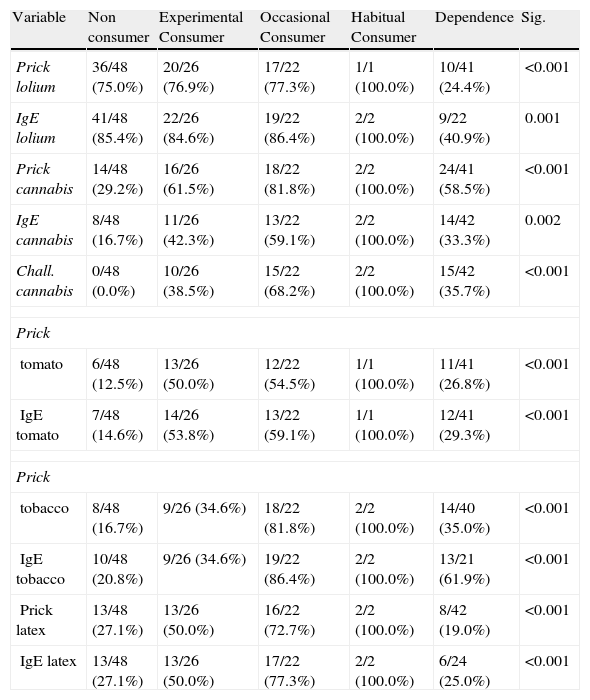
Differences by type of consumptionTable 5 shows positive prick and IgE test in study patients.
Greater cannabis consumption was associated with smoking and consumption of cocaine, heroin and alcohol (p<0.001). Anaphylaxis and ED attendance (p<0.001) was observed in experimental and occasional users. (Table 4)
Differences in clinical variables according to level of cannabis consumption.
| Variable | Non consumer n=48 | Experimental Consumer n=26 | Occasional Consumer n=22 | Habitual Consumer n=2 | Dependence n=42 | Sig. |
| Atopy | 48 (100.0%) | 26 (100.0%) | 22 (100.0%) | 2 (100.0%) | 29 (69.0%) | <0.001 |
| Asthma | 42 (87.5%) | 19 (73.1%) | 18 (81.8%) | 2 (100.0%) | 18 (42.9%) | <0.001 |
| Rhinitis | 14 (29.2%) | 0 (0.0%) | 0 (0.0%) | 1 (50.0%) | 11 (26.2%) | <0.001 |
| Anaphylaxis | 2 (4.2%) | 6 (23.1%) | 8 (36.4%) | 1 (50.0%) | 6 (14.3%) | 0.006 |
| Urticaria | 9 (18.8%) | 8 (30.8%) | 1 (4.5%) | 0 (0.0%) | 9 (21.4%) | NS |
| OAS | 8 (16.7%) | 15 (57.7%) | 11 (50.0%) | 1 (50.0%) | 15 (35.7%) | 0.004 |
| Tomato Intol. | 8 (16.7%) | 15 (57.7%) | 13 (59.1%) | 2 (100.0%) | 15 (35.7%) | <0.001 |
| Emergency department | 0 (0.0%) | 7 (26.9%) | 11 (50.0%) | 2 (100.0%) | 31 (73.8%) | <0.001 |
OAS: Oral allergy syndrome. Intol: Intolerance.
The highest levels of positive cannabis prick tests and specific IgE were observed in habitual and dependent users (p<0.001). There were no differences between experimental and occasional users in the positivity of tests. There were no significant differences in the number of positive results according to the type of consumption (Table 5).
Differences in positive prick (area≥19mm2) and IgE (≥0.35kU/L) tests according to level of cannabis consumption.
| Variable | Non consumer | Experimental Consumer | Occasional Consumer | Habitual Consumer | Dependence | Sig. |
| Prick lolium | 36/48 (75.0%) | 20/26 (76.9%) | 17/22 (77.3%) | 1/1 (100.0%) | 10/41 (24.4%) | <0.001 |
| IgE lolium | 41/48 (85.4%) | 22/26 (84.6%) | 19/22 (86.4%) | 2/2 (100.0%) | 9/22 (40.9%) | 0.001 |
| Prick cannabis | 14/48 (29.2%) | 16/26 (61.5%) | 18/22 (81.8%) | 2/2 (100.0%) | 24/41 (58.5%) | <0.001 |
| IgE cannabis | 8/48 (16.7%) | 11/26 (42.3%) | 13/22 (59.1%) | 2/2 (100.0%) | 14/42 (33.3%) | 0.002 |
| Chall. cannabis | 0/48 (0.0%) | 10/26 (38.5%) | 15/22 (68.2%) | 2/2 (100.0%) | 15/42 (35.7%) | <0.001 |
| Prick | ||||||
| tomato | 6/48 (12.5%) | 13/26 (50.0%) | 12/22 (54.5%) | 1/1 (100.0%) | 11/41 (26.8%) | <0.001 |
| IgE tomato | 7/48 (14.6%) | 14/26 (53.8%) | 13/22 (59.1%) | 1/1 (100.0%) | 12/41 (29.3%) | <0.001 |
| Prick | ||||||
| tobacco | 8/48 (16.7%) | 9/26 (34.6%) | 18/22 (81.8%) | 2/2 (100.0%) | 14/40 (35.0%) | <0.001 |
| IgE tobacco | 10/48 (20.8%) | 9/26 (34.6%) | 19/22 (86.4%) | 2/2 (100.0%) | 13/21 (61.9%) | <0.001 |
| Prick latex | 13/48 (27.1%) | 13/26 (50.0%) | 16/22 (72.7%) | 2/2 (100.0%) | 8/42 (19.0%) | <0.001 |
| IgE latex | 13/48 (27.1%) | 13/26 (50.0%) | 17/22 (77.3%) | 2/2 (100.0%) | 6/24 (25.0%) | <0.001 |
Chall: Challenge.
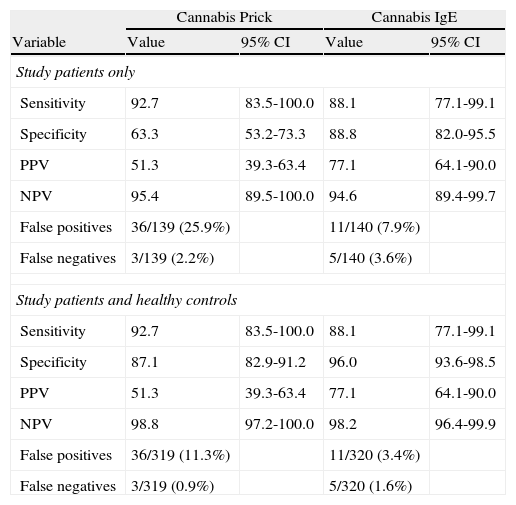
In study patients, the cannabis prick test had the highest sensitivity (92.7%) but a lower specificity, due to cross-reactivity with vegetable allergens, while specific IgE had a sensitivity of 88.1% and a specificity of 88.8%.
If controls (none of whom were positive for cannabis prick test, IgE, or challenge) were included, prick test sensitivity remained equal, but specificity increased, while IgE sensitivity and specificity remained high. (Table 6)
Sensitivity, specificity, positive and negative predictive values, false positives and false negatives of cannabis tests.
| Cannabis Prick | Cannabis IgE | |||
| Variable | Value | 95% CI | Value | 95% CI |
| Study patients only | ||||
| Sensitivity | 92.7 | 83.5-100.0 | 88.1 | 77.1-99.1 |
| Specificity | 63.3 | 53.2-73.3 | 88.8 | 82.0-95.5 |
| PPV | 51.3 | 39.3-63.4 | 77.1 | 64.1-90.0 |
| NPV | 95.4 | 89.5-100.0 | 94.6 | 89.4-99.7 |
| False positives | 36/139 (25.9%) | 11/140 (7.9%) | ||
| False negatives | 3/139 (2.2%) | 5/140 (3.6%) | ||
| Study patients and healthy controls | ||||
| Sensitivity | 92.7 | 83.5-100.0 | 88.1 | 77.1-99.1 |
| Specificity | 87.1 | 82.9-91.2 | 96.0 | 93.6-98.5 |
| PPV | 51.3 | 39.3-63.4 | 77.1 | 64.1-90.0 |
| NPV | 98.8 | 97.2-100.0 | 98.2 | 96.4-99.9 |
| False positives | 36/319 (11.3%) | 11/320 (3.4%) | ||
| False negatives | 3/319 (0.9%) | 5/320 (1.6%) | ||
Prick area ≥19mm2 and IgE≥0.35kU/L versus positive bronchial challenge test.
PPV: Positive predictive area.
NPV: Negative predictive area.
- 1.
After ACLAD patients, the highest level of use of addictive substances was in patients allergic to tobacco (p<0.001). However, the group with the highest level of sensitisation according to the prick test and specific IgE were patients allergic to tomato (p<0.001), although patients allergic to tobacco had the highest level of positive cannabis challenge (p<0.001), suggesting cross-sensitisation to cannabis and tomato and also that there may be true sensitisation to cannabis and false sensitisation due to cross-reactivity in patients sensitised to tomato.
- 2.
In addition to patients allergic to tomato, a greater or lesser degree of tomato intolerance was observed in half the patients allergic to tobacco and 15/42 ACLAD patients, all with positive cannabis challenge.
- 3.
The area of the cannabis prick test was greater in ACLAD patients (p<0.001) but levels of cannabis-specific IgE were higher in patients allergic to tomato (p<0.001).
- 4.
Intolerance to tomato should lead to the suspicion of cannabis consumption (p<0.001).
- 5.
Neither the severity of the clinical profiles nor ED admission was related to the level of consumption, with occasional users also being a risk group.
- 6.
Both the prick test and specific IgE were efficient in detecting sensitisation to cannabis and positivity was related to severe clinical profiles (anaphylaxis, asthma, angio-oedema) requiring ED admission.
It is estimated that in 2008 cannabis was consumed by 3.9% of the world population aged 15-64 years.17 Recent epidemiological studies2 show that regular cannabis use in adolescence causes adverse effects, including dependence syndrome, respiratory and cardiovascular alterations, increased traffic accidents, and alterations in psychosocial development and mental health. The possible adverse allergic effects have been very infrequently studied.
Isolated cases of allergic hypersensitivity to Cannabis sativa have been described, including rhinoconjunctivitis due to inhalation of cannabis pollen,3 urticaria and contact dermatitis due to handling of cannabis plants,4,5 and anaphylaxis due to cannabis ingestion.6 Paradoxically, the protective action of cannabis against allergies has also been described.7 Cross-reactivity between cannabis and tomato allergens8 and skin lesions due to inhalation of cannabis have been reported.9
Allergic hypersensitivity to tobacco has been demonstrated by our group in a large series of patients.11,12Cannabis challenge was positive in 52% of our patients sensitised to tomato and 61% sensitised to tobacco and both seem to be risk factors for cannabis sensitisation. ED admissions were due to severe symptoms related to probable multi-drug consumption. Patients with anaphylaxis were allergic to tomato and tobacco. Patients not attending the ED in spite of severe symptoms were younger (p<0.001) and possibly trying to hide their behaviour.
Might cannabis be an allergen?Reported cases are anecdotal and related to cannabis pollen inhalation. There are no reports of allergic hypersensitivity in drug abusers, who are normally excluded from clinical trials or studies, and usually receive no care for allergies, which are considered a minor problem. We felt a large study to highlight this health problem was warranted. This clinical study is based on routine allergy techniques, but we hope future studies will include new techniques of allergen analysis and cross-reactivity based on molecular biology and array techniques. The main strength of our study is that there is little attention in the literature to allergy associated with cannabis, even though about 4% of the population use this drug.
ED admissions for cannabis consumption have quadrupled in the last decade, from 7.4% to 28%.1,2 However, cannabis consumption has decreased by 6.8% since 2004.17 Drug abusers are usually attended by primary health care centres and drug rehabilitation centres and are rarely referred to outpatient allergy clinics but often admitted to the ED. The adverse effects of drug consumption are often not stated by intoxicated patients.
Once the possibility of allergic sensitisation to drugs is considered, new routes of laboratory detection of drug seem possible even when there is no current consumption, as the allergic reaction lasts over time and can be detected by specific antibodies. This simple, sensitive and objective method could have important social, legal and therapeutic repercussions:
- 1.
Social repercussions: Explaining that patients have an allergic reaction to the drug that may worsen progressively could be another argument in persuading them to reject drugs.
- 2.
Legal repercussions: Drug consumption could be detected even when there is no current consumption as the antibodies can be detected for some years.
- 3.
Therapeutic repercussions: In addition to avoiding fatal cases of anaphylaxis, specific immunotherapy, which is highly efficient for toxic allergens such as hymenopter toxins, may be possible. If the allergenic epitopes coincided with A-9-tetrahydrocannabinol (THC), specific immunotherapy against these allergens might be possible, using blocking antibodies which, theoretically, would annul the effect of the drug by blocking cannabinoid receptors and achieving tolerance to the allergen, presumably resulting in reduced consumption. The greater diagnostic yield obtained with cannabis bud extracts, which contain the greatest concentration of THC, would support this theory.
Strikingly, we found considerable cannabis consumption in a randomly-selected group of allergic patients. Studies in the USA have found that 10% of people who have ever consumed cannabis end up consuming it daily1 and, therefore, the risk of experimental consumption, especially in young students, should not be underestimated. According to our results, smokers and patients with difficulties ingesting tomato are also at risk. Although tomatoes possess allergens with cross-reactivity with cannabis allergens, 72% of patients with tomato allergy admitted cannabis consumption and the 13 patients with positive challenge had symptoms when they smoked it. Smokers sensitised to tobacco also responded to challenge with pure extract of cannabis leaf, showing that not only tobacco provoked their symptoms. Of the ACLAD patients, who had the highest rates of smoking, and consumption of alcohol and other drugs, 29/42 patients had atopy, an incidence greater than in the general public. Alcohol is reported to increase atopy and sensitisation to allergens.18
The effect of cannabis on pulmonary function is not clear.16 Habitual users have more symptoms of bronchitis and respiratory infections. Cannabis does not seem to increase the risk of emphysema19,20 and obstructive patterns improved in some of our patients after smoking cannabis. An Arizona study21 showed that 70% of atopic patients responded to marijuana pollen skin test, a habitual contaminating pollen in Arizona that provokes asthma, rhinitis and urticaria in sensitised patients, suggesting the opportunity for specific immunotherapy. However, there are few later references to possible cannabis allergy2–8 or specific immunotherapy.
We found the prick test highly-sensitive, although the diagnostic yield was due to a high negative predictive value. The papule area (19mm2, diameter 5x5mm), eliminated more false positives, improving the AOC. Specific IgE determination is a highly-sensitive and specific diagnostic parameter, with a better positive predictive value, and could be an easy, cheap and useful way of determining current or past contact with cannabis, and might one day be used for medical and legal ends (traffic accidents, behavioural alterations, violence).
In conclusion, cannabis consumption may be associated with a measurable allergic response. Determination of cannabis-specific IgE is a rapid, inexpensive method with high sensitivity and specificity that could detection consumption, sensitisation and possible allergy to cannabis and, perhaps, other illicit drugs.
Conflict of interestThe authors report no conflict of interest.
Declaration of all sources of fundingThis work was subsidised by the General Direction of Public Health, Research, Innovation and Development, Sanidad Castilla y León. (SACYL) and registered in its data base SACYL29022009.
We sincerely thank our patients, especially the ACLAD patients, for their courage and interest. They deserve our best efforts in the fight to overcome their addiction. Many ACLAD patients were on methadone and also had other problems (prostitution, sexually-transmitted diseases, hepatitis, AIDS). However, they were very receptive to any positive health intervention on their behalf.
The authors thank David Buss for his editorial assistance.