With the exception of tilapia tropomyosin, other anecdotic reports of tropomyosin recognition of vertebrate origin are generally not accompanied by clinical significance and a dogmatic idea is generally accepted about the inexistence of allergenicity of vertebrate tropomyosins, based mainly on sequence similarity evaluations with human tropomyosins. Recently, a specific work-up of a tropomyosin sensitised patient with seafood allergy, demonstrated that the IgE-recognition of tropomyosin from different fish species can be clinically relevant. We hypothesise that some vertebrate tropomyosins could be relevant allergens. The hypothesis is based on the molecular evolution of the proteins and it was tested by in silico methods. Fish, which are primitive vertebrates, could have tropomyosins similar to those of invertebrates. If the hypothesis is confirmed, tropomyosin should be included in different allergy diagnosis tools to improve the medical protocols and management of patients with digestive or cutaneous symptoms after fish intake.
Tropomyosins are a large family of fibrous proteins which are found in muscle and non-muscle cells. They stabilise and regulate the actin cytoskeleton which is involved in many cellular functions such as motility, division, contraction, signalling, transcription and intracellular transport.1 Plenty of tropomyosin isoforms are expressed in opisthokonts, a broad phylogenetic group of eukaryotes including both the animal and fungus kingdoms. However, it is not found in plants, protists or procaryotes.2 Cross-reactivity among tropomyosins from invertebrates is the reason for being considered invertebrate panallergens.3 Sequence identity of more than 50% between allergens seems to be necessary to exhibit cross-reactivity.4 Vertebrate tropomyosins share more than 50% of the protein sequence with that of invertebrates but, in contrast, they are not recognised by the IgE isotype.3 In a study on allergenicity of conserved proteins it was found that the higher the identity level with a particular organism's homologue, the lower the IgE prevalence in the population. Above 40% of sequence identity with human proteins, the number of allergens and the allergenicity decreased.5 Tropomysosin is one of the few exceptions, where sequence identity was, as was previously mentioned, above 50%. More specifically, vertebrate tropomyosins from mammals, birds and fish are at least 90% identical with at least one human tropomyosin.6 These data support the idea that vertebrate tropomyosins should not be allergenic. Some authors claimed that meat tropomyosins (Mammalia and Aves: cow, pig, chicken and rabbit) are not important allergens.7
The first vertebrate tropomyosin to be characterised and identified as allergenic at the WHO/IUIS Allergen Nomenclature Database (www.allergen.org) was Ore m 4, the Mozambique tilapia (Oreochromis mossambicus) tropomyosin.8 Furthermore, some other fish tropomyosins have recently been described as allergenic for a seafood allergic patient.9
HypothesisThe hypothesis is based on the molecular evolution of the proteins. Not all regions of the vertebrates chromosomes have the same substitution rates10 and not all vertebrate species have the same evolutionary time.11 Tropomyosin is an evolutionarily conserved protein but there are some amino acidic changes among different vertebrate species.12 Invertebrate species are phylogenetically far away from humans. However, within vertebrates, different fish species (which are primitive vertebrates) could have tropomyosins with characteristics more similar to the invertebrates’ ones and therefore be more likely to be allergenic.
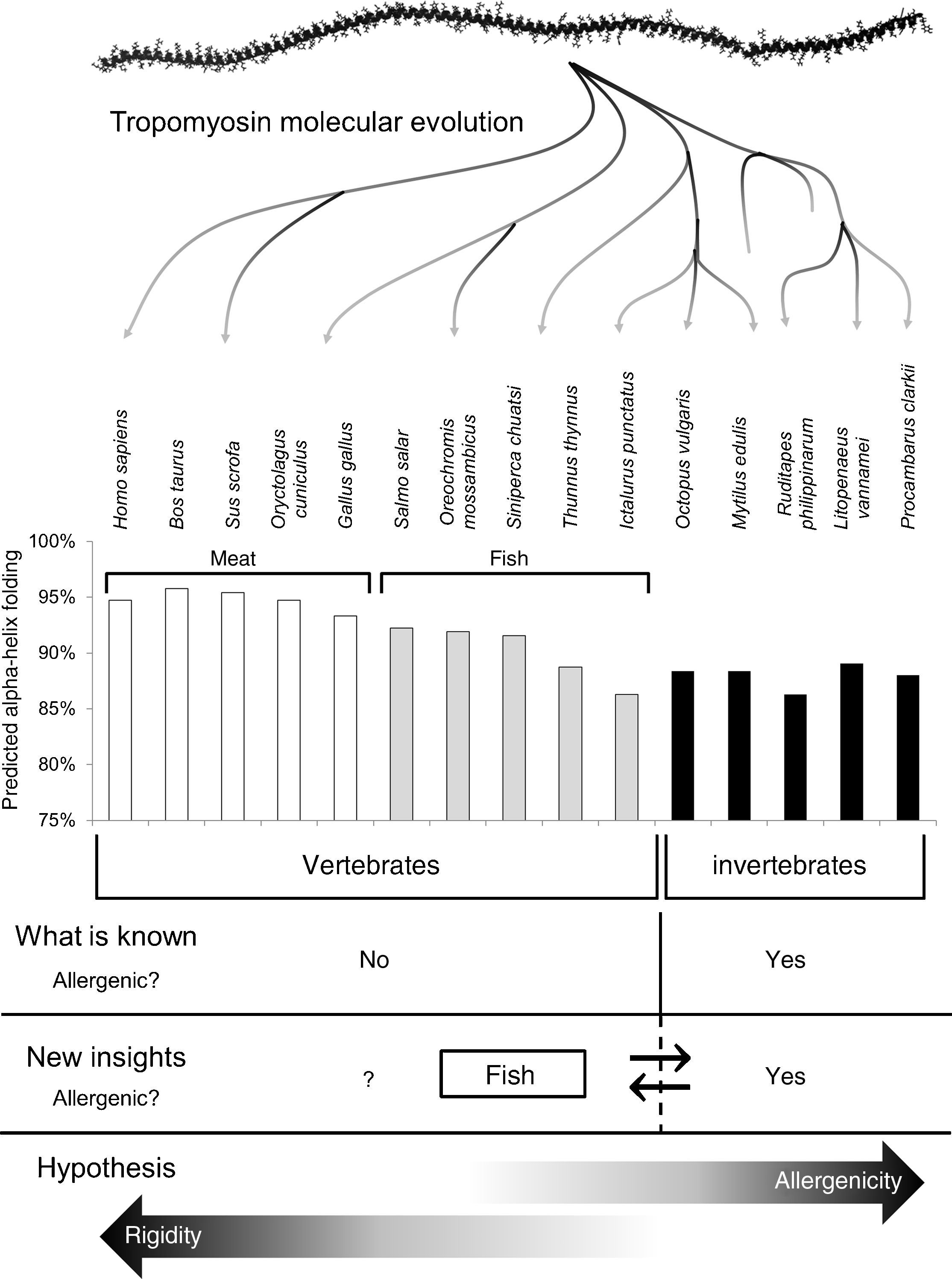
Invertebrates and fish (also reptiles and amphibians) share their ability to live with considerable variations in their internal temperature (poikilotherms). They are also ectotherms because they depend on external heat sources to regulate their body temperature. Allergenicity of fish tropomyosins could be due to the evolutionary fact that fish can live in cold water and their tropomyosins have strategical amino acid substitutions that solve the muscle rigidity induced by cold.13 This flexibility, as was previously hypothesised to be the case for tropomyosins from invertebrates,12 may confer epitopes that are not shown in tropomyosins from homeotherms (Fig. 1).
Schematic representation of the current tropomyosin allergenicity dogma, new insights of the tropomyosin allergenicity and our hypothesis. Prediction of alpha-helix folding was carried out as per González-Fernández et al. (2014)12 choosing edible species and including new fish tropomyosin with the following GenBank accession numbers: NP_001117128.1 Salmo salar; AFV53352.1 Oreochromis mossambicus; AEK21799.1 Siniperca chuatsi and NP_001187627.1 Ictalurus punctatus. The time-scale from invertebrates to humans is not linear.
Allergy literature has shown vertebrate tropomyosins as non-allergenic proteins. Nevertheless, in recent years new articles that are being published allow us to challenge this dogma.
Some basic questions should guide and help us when interpreting the recent scientific evidence:
- 1.
Why are some tropomyosins allergenic and others are not?
- 2.
Which are the differences between vertebrate and invertebrate tropomyosins?
- 3.
Are there novel scientific approaches which help us to elucidate our question?
- 4.
When are IgE antibodies clinically relevant?
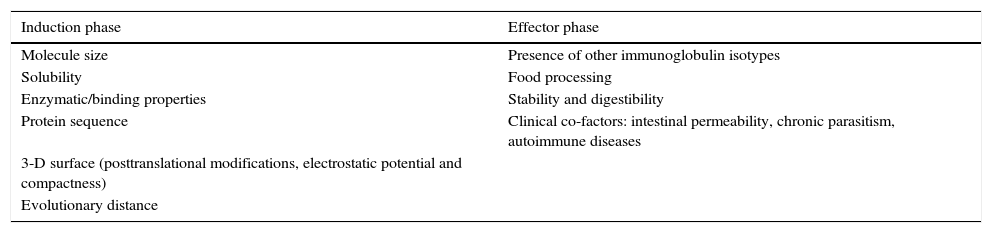
Classically the allergic immune reaction is divided into the induction phase and the effector phase. Thus, IgE production needs an initial allergen processing in a T helper 2 (Th2) biased milieu, encompassing allergen processing, a further Th2-response expansion and mast-cell sensitisation. Whereas many studies deal with the factors affecting the production of IgE, the clinical relevance of IgE antibodies can only be assessed in clinical observations or studies. Known and hypothetical factors affecting allergenicity of tropomyosins from different sources separated by those affecting primarily the induction or the effector phase are listed in Table 1. Besides general features affecting IgE recognition, evolutionary distance from human homologues seems by far to be the most straightforward factor and has been used to explain allergenicity of proteins.6
Known and hypothetical factors affecting allergenicity of tropomyosins from different sources.
| Induction phase | Effector phase |
|---|---|
| Molecule size | Presence of other immunoglobulin isotypes |
| Solubility | Food processing |
| Enzymatic/binding properties | Stability and digestibility |
| Protein sequence | Clinical co-factors: intestinal permeability, chronic parasitism, autoimmune diseases |
| 3-D surface (posttranslational modifications, electrostatic potential and compactness) | |
| Evolutionary distance |
We will show that evolutionary distance is a necessary, but not sufficient factor for explaining vertebrate IgE tropomyosin recognition.
Factors affecting tropomyosin allergenicityImmunoglobulin protective isotypesStudies on parasitism have helped us to understand why allergic phenomena are rare even in high IgE producing situations. Chronic parasitism by helminths induces not only IgE, but also a parallel anti-inflammatory axis, which dampens the allergic expression. One of the proposed mechanisms is the production of other blocking isotypes, such as IgG4 or IgA in much higher quantities. In human anisakiasis, specific IgG4 antibodies have been shown to be produced in parallel to IgE, hindering Anisakis allergens to behave like food allergens14. IgG4 blocking antibodies against Schistosoma japonicum paramyosin are associated with susceptibility to reinfection.15 Parasites induce simultaneous IgE and IgG4 antibody responses but only a positive balance of IgE offers protection against the worms.16 Some authors claimed that IgA antibodies against Schistosoma antigens could participate in the protective immune response against schistosomiasis inducing inhibition of egg laying.17
On the other hand, in food allergy, it is not clear which factors contribute to a parallel production of other specific isotypes. Oral sensitisation with purified tropomyosin from shrimp Metapenaeus ensis, induced significant levels of serum IgE, IgG1 (equivalent to IgG4 isotype in humans) and faecal IgA in C3H/HeJ mice.18
Food processingThe processing of the food is relevant when assessing the clinical relevance of allergens. Due to the fact that tropomyosin is the major shellfish allergen, different food processing methods have recently been investigated, such as heat, high pressure, ultrasound, and gamma irradiation.19
Heating showed contradicting results, in some studies the heated tropomyosins seem to be more allergenic but in other cases a decreased IgE binding has been detected. Maybe the reason is that tropomyosin can refold upon cooling after a heat denaturing process.20 We consider that IgE binding epitopes can sometimes be masked with other components of the extract or new epitopes may be formed when tropomyosins aggregate. Likewise, dimer formation of tropomyosin molecules could induce a different profile of class immunoglobulin major production. Actually, the two isoforms of the major birch pollen allergen (Bet v 1a and Bet v 1d) induced similar specific IgE levels but only Bet v 1d induced serum IgG1, IgG2a and IgA.21
High pressure processing was developed to maintain sensory and nutritional properties in food while inactivating microorganisms’ growth. This physical treatment affects non-covalent bonds of protein structures. A recent study about horse mackerel high-pressure processing showed a significant degradation of some proteins and an increased intensity of the tropomyosin after applying 300 and 450MPa.22 This fact could also be due to aggregation of different molecular weight isoforms or fragments from other proteins and might be hiding or showing new epitopes.
Ultrasound and gamma irradiation are very effective in combination with heat to decrease the IgE binding to the tropomyosin of Litopenaeus vannamei.19
Stability and digestibilityFurthermore, digestibility of the different tropomyosins according to their origins could account for their allergenicity. Some authors claimed that invertebrate tropomyosins result in longer fragments after in silico digestion and this could be the reason for the allergenicity23 but experimentally mud crab tropomyosin was resistant to pepsin and relatively susceptible to trypsin and chymotrypsin digestion.24 Finally, different experiments testing the digestibility of shellfish tropomyosin showed that combinations with high pressure boiling and ultrasonic treatment could accelerate the digestion.19 Recombinant fragments of shrimp tropomyosin Pan b 1 were even recognised, especially the terminal and the middle ones, with the allergenicity of the protein not being reduced.25
Clinical co-factors: intestinal permeability, autoimmune diseasesThere is a known link between intestinal permeability and allergic disease or inflammatory bowel disease with clinically relevant vertebrate tropomyosin IgE antibodies.8 Allergenic tilapia tropomyosin shares identical amino acids with the human tropomyosin isoform 5 against which ulcerative colitis patients have IgG auto-antibodies. The allergenicity link with the intestine could however be due to the above-mentioned differences of digestibility of the different tropomyosins according to their origins.23
Evaluating the hypothesisWe will evaluate the hypothesis on the one hand in the light of recent experimental and clinical studies that suggest that fish tropomyosins could be allergenic, and on the other hand pointing out interesting in silico approaches that show tropomyosin as a protein, whose sequence has changed more slowly over evolutionary time because of its cellular function importance, and is therefore highly similar to the human one (but not identical). Therefore, with this evaluation we would like to merge experimental/clinical and bioinformatics evidences to give importance to three dimensional features that could be modifying the IgE binding epitopes and consequently the allergic response of vertebrate tropomyosins, especially from fish tropomyosins.
Experimental and clinical newsThere are two main experimental articles that support our hypothesis. The first can be considered the breaking of the dogma by describing the first allergenic vertebrate tropomyosin of tilapia (Ore m 4).8 The authors claimed that the amount of the anti-tropomyosin IgE did not significantly correlate with severity of symptoms of the 10 patients studied. Allergic and digestive symptoms were observed in patients after a positive double-blind placebo-controlled food challenge with tilapia. In general, if some tropomyosins reach the several necessary steps to induce an IgE response, the clinical relevance will highly depend on other factors, such as the administration route or concomitant protective factors, such as the existence of IgG4 or IgA antibodies.14 Indeed, in the tilapia study it is stated that no patient had IgG against this tropomyosin. A previous diagnostic of fish allergy was directly related with the onset of inflammatory bowel disease (IBD) after tilapia intake. However, four out of 10 tilapia allergic patients had concomitant shrimp allergy and these four patients did not show IBD after eating tilapia. Interestingly, shrimp tropomyosin inhibits approx. 55% of serum IgE binding on the epitopes of Ore m 4, so there are cross-reacting epitopes with shrimp tropomyosin but part of the recognition is due to fish specific tropomyosin epitopes.8
Recently, a specific work-up of a tropomyosin sensitised patient with seafood allergy demonstrated that the IgE-recognition of tropomyosin from different fish species can be clinically relevant.9 In this report, the patient also recognised tropomyosins of molluscs, arthropods (crustaceans, mites and cockroaches) and nematodes (Anisakis, Ascaris and Trichinella) in the absence of parasitosis, and was clinically relevant. At the age of 13 he began having symptoms after intake of several fish species. IgE, IgG4 and IgA immunorecognition profiles were carried out against 13 species of fish. IgE was detected in four out of six species against which the patient had symptoms, so tropomyosin of these fish (European seabass, albacore, swordfish and European hake) are cross-reactive. Interestingly, he tolerated canned sardines, anchovies or tuna as well as smoked or cooked salmon, which are, except cooked salmon, processed fish. Concerning protective antibodies, IgG4 isotype was not detected against any fish. Even though he presented IgA against Atlantic Bluefin tuna he also presented this isotype against other organisms that he could not tolerate.
In both articles we found cross-reactive vertebrate tropomyosins with invertebrate tropomyosins and symptoms after fish intake in the absence of protective immunoglobulins.
On the other hand, the classical view of vertebrate tropomyosins as non-allergenic was confirmed principally in meat.7 By means of dot blot using cow, pig, chicken and rabbit tropomyosins, only two out of 57 patients were positive to tropomyosin (patient 53 to the chicken and patient 41 to pork tropomyosin). However, soft shadows can be observed for chicken tropomyosin also in patients 24, 32 and 45 (in addition to patient 53). Furthermore, patients 24, 32, 45 and 53 seem to have the same IgE immunoblotting profile against chicken. In addition, amongst the 57 meat allergic patients just three reported symptoms with pork and 23 with chicken so, the importance of tropomyosin could be higher in the case of the pork.
Regarding fish, parvalbumins continue being the major allergens. Nevertheless, many others have been discovered as fish enolases, aldolases and fish gelatine as well as unidentified proteins.26 Beta-prime component of vitellogenin of chum salmon caviar has also been added to IUIS allergen database and the above mentioned tilapia tropomyosin. In fish allergic patients we still cannot claim a role of tropomyosin as a potential panallergen because the only two existing studies, on which the hypothesis is based, are not enough. Further studies with more fish allergic patients must be carried out to check if tropomyosin is involved in fish allergy on a wider scale.
In silico evidenceNonetheless, the new experimental and clinical data leave us without an answer as to why a tropomyosin molecule with such a high similarity with human tropomyosins should be immunogenic, irrespective of the produced isotype. One approach could be the evaluation of a possible autoimmunity, such as the anti-tropomyosin antibodies produced in the scenario of ulcerative colitis, Behçet's disease, Alzheimer's disease and autoimmune hepatitis.27 The other approach is to highlight other independent amino acid sequence similarity assessment.
As was mentioned in our hypothesis, amongst the vertebrates, fish are closer to the invertebrates in the phylogenetic tree. This should generally be in accordance with a higher probability of allergenicity compared to meat tropomyosins. But it has been described that molecular uniqueness rather than molecular similarity between allergens and human proteins, underlies allergic responsiveness to environmental proteins5.
Furthermore, conformational issues of proteins are of extreme importance and could explain minor differences in amino acid sequences to reflect the loss of allergenicity.7 An in silico study showed that alpha-helix folding structure of the vertebrate tropomyosins could be responsible for the lack of allergenicity. In addition, it was claimed that tuna tropomyosin share a similar low probability of alpha-helix folding as invertebrate tropomyosins.12 Another new in silico study has been carried out showing a common shrimp (invertebrate) tropomyosin linear epitope in the channel catfish (Ictalurus punctatus) (vertebrate) tropomyosin.28 These authors claimed that a protein that contains linear epitopes from other allergens in their sequences is candidate to be allergenic but the absence of known epitopes does not ensure that this protein is not an allergen. What is extremely interesting is that, both in silico studies consider the fish tropomyosins as candidates to be allergens.
In addition, it is worth mentioning that most IgE epitopes are conformational.29In silico allergenicity predictions with other conserved allergens such as haemoglobin30 have recently been carried out. However, conformational (B-cell) epitopes have not been studied on the tropomyosin molecule because of its simple alpha-helix folding. In fact, there are common B-cell epitopes in tropomyosins from vertebrates and invertebrates as was confirmed in a previous study with the monoclonal antibody anti-tropomyosin N11 of the filarial parasite Achantocheilonema viteae that recognised also tropomyosin molecules from: octopus, perch and chicken (with a strong binding), and Alaska Pollock, salmon, sparrow, pig and mouse.31
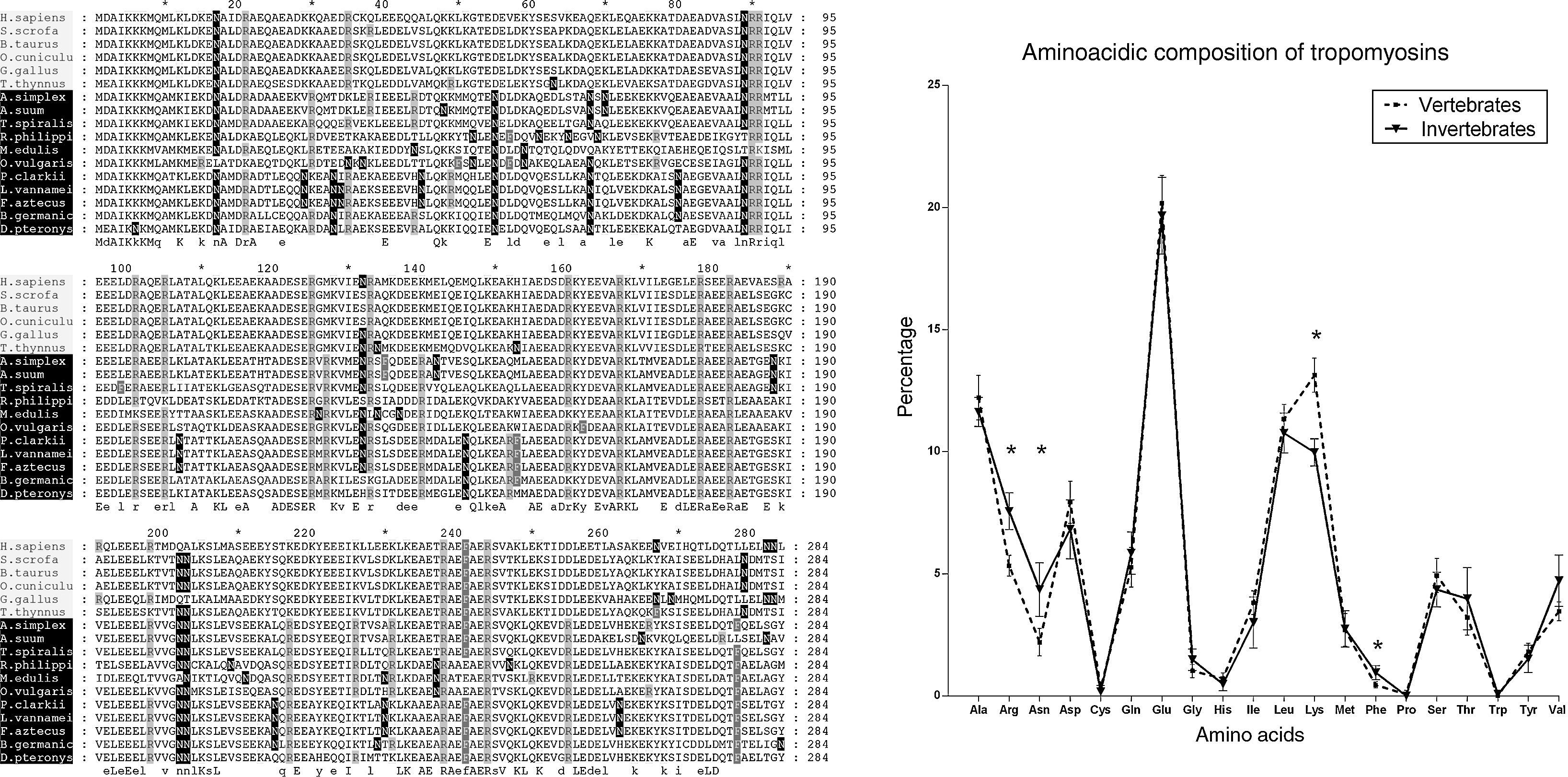
Studying the primary structure of the tropomyosins in silico, we could appreciate significant differences in the proportions of lysine-K, arginine-R, phenylalanine-F and asparagine-N between the groups of vertebrates and invertebrates (Fig. 2) when we analysed the amino acidic composition of the tropomyosins from a previous study.12 In addition, the apparently simple alpha-helix folding of tropomyosins has been crucial to identify differences between vertebrates and invertebrates looking at the secondary structure.12 However, it is necessary to assess one further step of complexity because the most important aspect is the three-dimensional surface of the protein. The amino acids have a specific side-chain, which interacts with other neighbours’ groups from the same or from other proteins or carbohydrates and phosphates modifying the epitopes and consequently their recognition by the antibodies. This fact is, indeed, the key to understanding the allergic reaction. For instance, the differential proportion of asparagine lead us to think in the differences not only in the three-dimensional shape of the surface but also in the possible new N-glycosylation sites that are available in the invertebrate tropomyosins. The existence of an in silico method for predicting cross-reactions in panallergens based on the surface instead of the sequence similarity, would be extremely useful not only to improve our knowledge in the field but would also translate into practical issues, such as avoiding the specific food that induces allergy in the patients’ diet.
Alignment of tropomyosins analysed by González-Fernández et al. (2014)12, shading the amino acids with significant different proportion between the groups of vertebrate and invertebrate tropomyosins. The percentage of lysine-K was higher in vertebrate tropomyosins compared to the invertebrates ones (13.13% vs. 9.97%). The percentages of arginine-R (5.33% vs. 7.56%), asparagine-N (2.2% vs. 4.35%) and phenylalanine-F (0.45% vs. 0.95%), were significantly higher in invertebrate tropomyosins compared to the vertebrates. Vertebrate tropomyosins (N=6); invertebrate tropomyosins (N=11). Statistical test used: Student's t-test; * indicates p<0.01.
Meat allergy is rare and usually occurs in children. This could be the reason for the paucity of molecular studies and the few proteins identified as meat allergens. Nonetheless, fish allergy is present in up to 0.2% of the general population and the prevalence is increased in areas where there are more fish industries or the fish consumption is higher.26 There are more molecular studies with fish than with meat and new panallergens are being identified at present. This was the case of collagen, which is present in raw fish, but when it is heated denatures to gelatine. In Japan, fish is eaten raw and collagen is insoluble in this form, showing the major IgE-binding epitopes.32 This allergen was under-reported because of solubility problems with skin tests.33 In the same way, physicochemical factors, such as cross-linking, aggregation or interactions with fish fatty acids might be masking the role of tropomyosins as fish allergens.
Tropomyosin was also found in the epithelial mucus of gilthead seabream, Atlantic cod and common catfish.34 There is also a wide variety of bacteria including the genus Streptococcus on the gilthead seabream mucus.34 This brings up the interesting fact that S. pyogenes (group A streptococci) express tropomyosin-like proteins named M proteins. In particular, M5 and M6 are cross-reactive with human and rabbit tropomyosins. Although they share identical residues, these identities are not localised in a linear region due to the fact that both are coiled-coil structures and only external amino acids positions are shared35. S. pyogenes is a common pathogen but is also carried asymptomatically on the human skin and nasopharyngeal mucosa36. These tropomyosin-like proteins could be responsible for a primary sensitisation, also being a starting point for research in the field of food allergy.
The sequence similarity among tropomyosins indicates phylogenetic proximity but there are also modifications like glycosylations or phosphorylations that theoretically affect the three-dimensional surface of the protein in which the antibodies are going to bind. However, in the case of the crab tropomyosin, which is an O-glycosylated protein, the presence/absence of these carbohydrates does not seem to affect the allergenicity.37 Still, these modifications are different not only between the groups of vertebrates vs. invertebrates, but also among different species in each group. In the case of phosphorylations, Atlantic cod and shark skeletal muscle have phosphorylated tropomyosins whereas salmonids’ ones are unphosphorylated.38 This confers a specific surface and charge to each tropomyosin in each species or groups and a specific recognition by the allergic patients.
Summarising, we do not know exactly if detection of IgE against vertebrate tropomyosins is really unusual. We also do not know if the clinical symptoms could be due to this allergen or others because dogmatic view has hindered to consider tropomyosin as a potential allergen. Tropomyosins may appear as monomers or as different homomers at different molecular weights and there are many reports of allergenic bands recognised by meat and fish allergic patients without identifying them.26,39 Thus, we should not discard the idea of tropomyosin just for coming from a vertebrate source.
Several interesting studies have increased our knowledge about invertebrate tropomyosin allergenicity. There are some exceptions to the dogma showing unexpected vertebrate tropomyosin allergenicity:
- -
Ore m 4, the first fish and vertebrate allergenic tropomyosin.
- -
The case of a tropomyosin allergic patient who displayed clinical symptoms after eating different fish species against which serum anti-tropomyosin IgE was detected.
Both experimental and in silico evidence allowed us to establish the hypothesis of a possible allergenicity of the vertebrate tropomyosins. This could be a starting point to new research in allergy aiming the identification of more allergenic tropomyosins from other vertebrate sources in addition to design new tools for a better diagnosis of fish allergy.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestThe authors have no conflict of interest to declare.
JGF is supported by a PhD fellowship from Universidad Complutense de Madrid, Spain.