Rhinitis is a very common disease, frequently caused by sensitisation to inhalant allergens. Negative results from skin prick tests (SPT) and in vitro IgE tests generally lead to a diagnosis of non-allergic rhinitis. However, it is possible, as indicated by studies addressed with dust mites or pollens that the production of specific IgE occurs exclusively at nasal level.
MethodsWe measured specific nasal IgE in children suffering from rhinitis in the periods when Alternaria spores were present in the air.
All subjects underwent SPT with a standard panel of aeroallergens (Stallergenes, Milan, Italy) and, in the same session, to nasal IgE test (NT). Nasal provocation test (NPT) with Alternaria was used as reference.
ResultsFifty-six subjects were included in the study. Of them, 20 (37.5%) were positive to SPT and 45 (80.3%) were positive to NT. In particular, 11 subjects (19.6%) had a positive SPT and a negative NT; 36 (64.3%) had a negative SPT and a positive NT; and 9 (16.1%) were positive to both tests. Positivity of NT and NPT was observed in 36 patients (69.6%), while positivity of SPT and NPT was observed in 15 patients (26.8%). This difference was highly significant (p<0.0001).
ConclusionsThese findings suggest that sensitisation to Alternaria is frequently expressed by exclusive production of specific IgE in the nasal mucosa. Thus, measuring nasal IgE in children with rhinitis and negative SPT during the period of presence of Alternaria spores seems helpful to avoid a mistaken diagnosis of non-allergic rhinitis.
Respiratory allergy with rhinitis and/or asthma is caused by clinical sensitisation to environmental inhalant allergens. Among these allergens, the importance of fungal spores has long been scantily investigated. However, recent studies have demonstrated that the prevalence of fungal sensitisation is higher than believed. As regards studies conducted in Europe, a study sponsored by the Spanish Society of Allergy and Clinical Immunology found that 9.5% of patients who had been visited due to suspected respiratory allergy were found to be sensitised to Alternaria or Cladosporium.1 In the survey promoted by the European Academy of Allergy and Clinical Immunology (EAACI) and conducted in seven countries – Spain, Portugal, Italy, Greece, Austria, Switzerland, and Germany – the prevalence of positive tests to the two moulds ranged from 3% in Portugal to 20% in Spain.2 Other investigations were conducted in Finland, where a prevalence of positive skin prick test (SPT) corresponding to 2.8% for Alternaria and to 2.7% for Cladosporium were detected,3 and, very recently, in France. This latest study was addressed only to Alternaria sensitisation and included a population of 6,726 children, of whom 2.8% had a positive result to Alternaria and to other allergens, while 0.8% were mono-sensitised to Alternaria.4
The clinical expression of sensitisation to Alternaria may include rhinitis, asthma, or both, with rhinitis or asthma prevailing in the different studies.2–5 However, sensitisation in general is not always detected by SPT and in vitro IgE measurement. In fact, it has been long known6 and recently confirmed7,8 that some subjects with rhinitis and negative skin tests may have IgE only in the nasal mucosa, due to an exclusive local production.
We evaluated the local production of IgE by performing a nasal IgE test on children with rhinitis occurring during the period of the year when Alternaria spores were present.
MethodsPatientsFrom children referred for suspected allergy to our Paediatric Allergy Service, all subjects reporting rhinitis symptoms such as sneezing, nasal itching, rhinorrhoea, and nasal obstruction over a period of at least two years in the periods corresponding to the presence of Alternaria spores in our area – from the 3rd week of May to the 1st week of July and from the 3rd week of August to the 2nd week of October9 – were included in the study.
All patients underwent SPT with a standard panel of inhalant allergens (Stallergenes, Milan, Italy) and, in the same session, measurement of specific nasal IgE by an already validated10 commercial kit (Biomicron, Turin, Italy). Nasal provocation test with Alternaria was performed in December, when Alternaria spores were by then absent in the atmosphere.
All children's parents gave an informal consent.
Nasal IgE testNasal IgE test (NT) was performed by the technique originally described by Marcucci et al.11 In brief, the allergen coupled cellulose derivative was placed in a two-hole applicator strip covered with a permeable membrane (to avoid the adhesion of nasal mucus on the substrate) and positioned in the above-posterior tract of the internal ostium for 10minutes. The results were subsequently read according to a colorimetric reaction and expressed in a scale from 0 (negative) to 4 (highly positive), according to a calibration curve.
Nasal provocation test (NPT)NPT was performed using the Alternaria extract Alyostal (Stallergenes) titrated in 100 Index of Reactivity (IR). The test started by administering physiological solution in one nostril and then 100μl. of increasing concentrations of the extract every 20minutes alternating between both nostrils. The concentrations used were 0.2, 0.5, 0.8, 1.8, and 2.8 IR. After each administration the nasal symptoms (nasal itching and sneezing, runny nose, and nasal blockage) were evaluated by a scoring system attributing 1 point to mild, 2 points to moderate, and 3 points to severe symptoms.12
All NPTs were performed by the same operator (N.F.) in a symptom-free period when no drug which could have interfered with the nasal response to the specific allergen was being used.
Statistical analysisData are presented as percentage and as mean±sd for qualitative and quantitative variables, respectively. Statistical analysis was performed using χ2 test and Z test to compare the proportions of patients with NT / NPT positivity and SPT / NT positivity, respectively. Statistical significance was assumed for p<0.05 and, for Z test two tails.
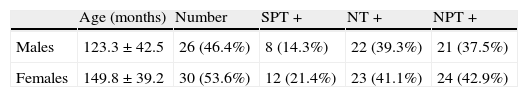
ResultsFifty-six children, 30 males and 26 females of age ranging between 50 and 216 months (mean age 137.5±43.2 months, median age 137.5 months) were included in the study. Table 1 shows the results of SPT and NT. Twenty subjects (37.5%) were positive to SPT and 45 (80.3%) were positive to NT. In particular, 11 subjects (19.6%) had a positive SPT and a negative NT; 36 (64.3%) had a negative SPT and a positive NT; and 9 (16.1%) were positive to both tests.
NPT was positive in all patients with double positivity to SPT and NT; in 6 of 11 patients (54.5%) with positive SPT and negative NT; and in 30 of 36 patients (83.3%) with negative SPT and positive NT. Positivity of NT and NPT was observed in 36 patients (69.6%), while positivity of SPT and NPT was observed in 15 patients (26.8%). This difference was statistically highly significant (p<0.0001).
DiscussionIgE antibodies are a key factor in hypersensitivity diseases13 and their detection by skin tests or by in vitro measurement reveals atopic sensitisation. When results of these tests are in agreement with history data, and above all when a cause-effect relationship with the exposure to given allergen(s) is apparent, the diagnosis of clinical allergy is achieved. However, it is not rare to observe subjects with rhinitis whose history of suspected allergy is not confirmed by IgE tests (performed with adequate material and optimal technique), and this commonly leads to a diagnosis of non-allergic rhinitis. In patients with seasonal symptoms occurring in correspondence to the presence in the air of known allergens, searching for allergy using other tests is warranted. In recent years, new in vitro tests assessing the cell reactivity to the allergen, such as the basophil activation test, have been introduced.14 However, their diagnostic value is still under evaluation.
Another approach to investigate the matter is to find the IgE antibodies directly in the nasal mucosa. The possibility of an exclusively local production of allergen-specific IgE antibodies was first demonstrated in 1975,6 subsequently confirmed,15 and recently reviewed by Rondon et al. who estimated that a local IgE production occurs in about 40% of the non-allergic rhinitis patients and proposed for such entity the definition of local allergic rhinitis.8 Of course, it must be demonstrated that the locally produced IgE are able to elicit symptoms, and this requires nasal symptoms to be reproduced by a NPT using the suspected allergen(s).
We used this approach to evaluate children with rhinitis symptoms during the months when Alternaria spores are present in their living area. Of the 56 children, 20 (37.5%) were positive to SPT, and 45 (80.3%) were positive to NT. We chose to use SPT instead of in vitro IgE measurement because SPT remains the most sensitive test even using the recent in vitro tests with improved performances16 The most interesting finding was that 36 subjects (64.3%) had a negative SPT and a positive NT. As expected, the NPT with the Alternaria extract was positive in all patients with double positivity to SPT and NT, but was also positive in 69% of patients with positive NT compared with 27% of those with positive SPT, this being a highly significant difference.
There are reports regarding the role of Alternaria in adult patients with nasal polyposis, as assessed by measurement of specific IgE in tissues homogenates of polyps17 or sinus tissues,18 but to the best of our knowledge there are no studies investigating the local production of specific IgE to Alternaria in patients with seasonal rhinitis. However, there is a possible relationship between the findings of our study in children and from the investigations on nasal polyposis in adults. In fact, the persistent exposure to the causative allergen in the absence of a clinical diagnosis and a consequent treatment may lead to rhinosinusal chronic inflammation and to the formation of nasal polyps.
Our finding that sensitisation to Alternaria is frequently expressed by exclusive production of specific IgE in the nasal mucosa suggests the need to perform a measurement of IgE in the nasal mucosa in subjects with seasonal rhinitis and negative SPT during the period of presence of Alternaria spores. The detection of specific IgE in the nose may be able to recognise the real causative agent and to avoid an incorrect diagnosis of non-allergic rhinitis.
Conflict of interestThe authors declare they have no conflict of interest.