Many new agents have been introduced as an alternative to standard MVAC therapy with improved efficacy and lower toxicity profile in advanced bladder carcinoma. The aim of this study is to evaluate the response rate and toxic side effects of gemcitabine–cisplatin (GC) in patients with advanced/metastatic bladder carcinoma.
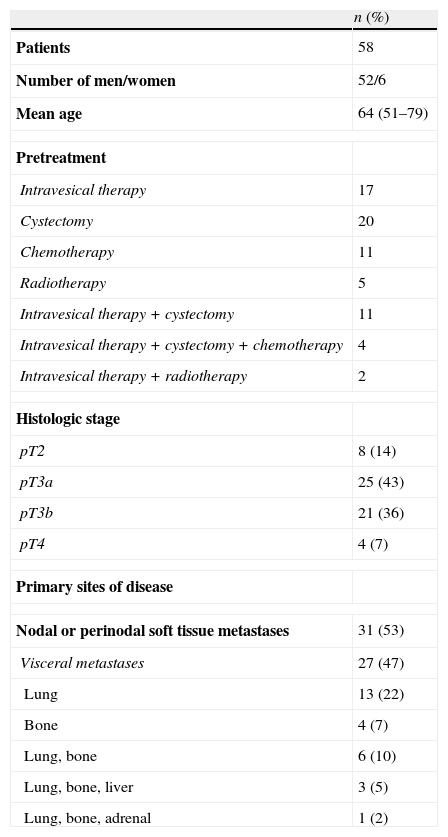
MethodsBetween January 2001 and April 2006, 58 patients with histologically confirmed advanced/metastatic transitional cell carcinoma (TCC) were enrolled in the study. All patients received 1000mg/m2 gemcitabine administered via intravenous infusion of 30–60min on days 1, 8 and 15, and 70mg/m2 cisplatin as an infusion of 60-min on day 2. All toxicities were graded using the WHO scale and the National Cancer Institute scale.
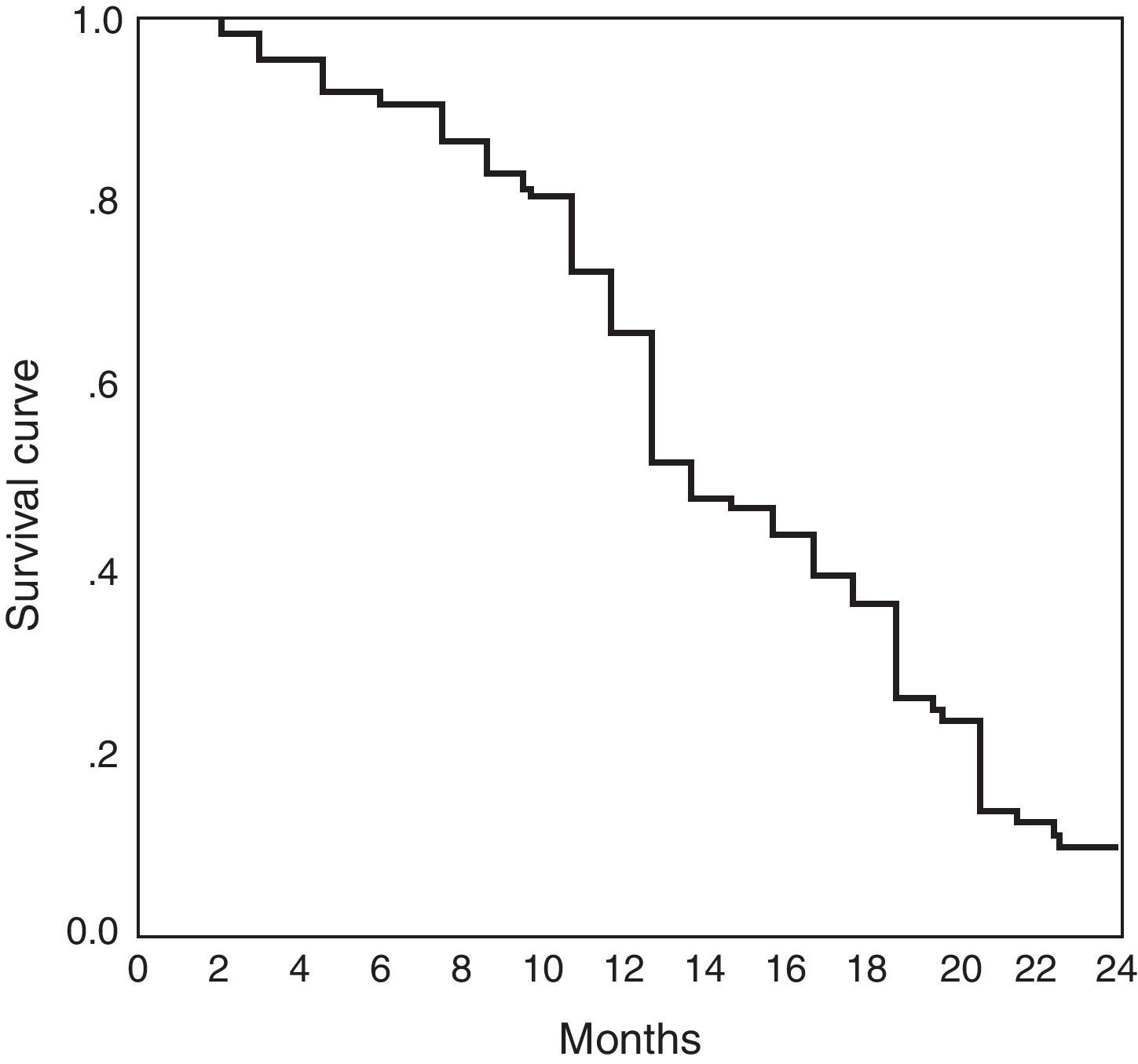
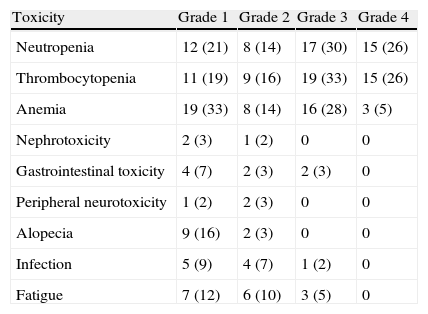
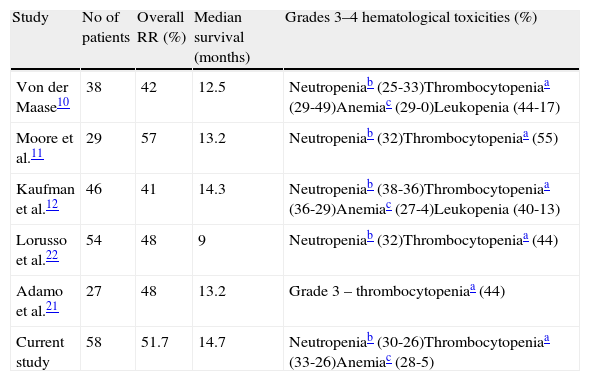
ResultsThe average number of cycles was 4.1. Neutropenia and thrombocytopenia were clinically significant treatment-related side-effects. Hematologic toxicity included mainly grades 3 and 4 neutropenia in 56%, grades 3 and 4 thrombocytopenia in 59%, and grades 3 and 4 anemia in 33% of patients. There was only one death from neutropenic sepsis. Complete response and partial response were obtained in 13 (22.4%) and 17 (29.3%) of patients, respectively, 17 (29.3%) of patients were found to have stable disease, and progression was observed in 11 patients (18.9%). Median survival for the whole group was 14.7 months (2–67).
ConclusionsGC therapy is an effective regimen owing to its high tumor response and long survival with a low incidence of toxicity in advanced or metastatic patients.
Se han introducido muchos agentes nuevos como una alternativa a la terapia MVAC estándar con eficacia mejorada y un menor perfil de toxicidad en el carcinoma de vejiga avanzado. El objetivo de este estudio es evaluar la tasa de respuesta y los efectos secundarios tóxicos de gemcitabina-cisplatino (GC) en pacientes con carcinoma de vejiga avanzado/metastásico.
MétodosEntre enero de 2001 y abril de 2006 58 pacientes con carcinoma de células transicionales (CCT) avanzado/metastásico histológicamente confirmado se inscribieron en el estudio. Todos los pacientes recibieron 1000mg/m2 de gemcitabina administrada mediante infusión intravenosa de 30-60 minutos los días 1, 8 y 15 y 70mg/m2 de cisplatino como una infusión de 60 minutos el día 2. Todas las toxicidades fueron clasificadas según la escala de la OMS y la del Instituto Nacional del Cáncer.
ResultadosEl número medio de ciclos fue de 4,1. La neutropenia y la trombocitopenia fueron efectos secundarios clínicamente significativos relacionados con el tratamiento. La toxicidad hematológica incluyó principalmente la neutropenia de grado 3-4 en el 56% de los pacientes, la trombocitopenia de grado 3-4 en el 59% y la anemia de grado 3-4 en el 33%. Solo hubo una muerte por sepsis neutropénica. La respuesta completa y respuesta parcial se obtuvieron en 13 (22,4%) y 17 (29,3%) pacientes, respectivamente; 17 (29,3%) de los pacientes tenían enfermedad estable y se observó progresión en 11 pacientes (18,9%). La mediana de supervivencia para todo el grupo fue de 14,7 meses (2-67).
ConclusionesLa terapia con GC es un tratamiento eficaz debido a su alta respuesta tumoral y la supervivencia a largo plazo, con una baja incidencia de toxicidad en pacientes avanzados o metastásicos.