Sunitinib (SUN) and Pazopanib (PAZ) are two oral tyrosine kinase inhibitors (TKIs) against vascular endothelial growth factor (VEGF). Their efficacy and safety in metastatic renal cell carcinoma (mRCC) has been proven with phase III studies. However, real world data is limited. The objective of this study is to assess the clinical benefit of SUN and PAZ in routine practice.
MethodsWe reviewed the medical records of seventy-nine mRCC patients treated with SUN (50 mg/day on 4/2-schedule) or PAZ (800 mg/day continuously). Patients were assessed retrospectively at two Turkish hospitals between 2006 and 2016.
ResultsFor the entire cohort median age of patients was 60 (28–87) years and 70% of them were male. The objective response rate (ORR) and disease control rate (DCR) in SUN/PAZ groups were 34/37% (p = 0.96) and 78/87% (p = 0.046), respectively. With a median follow up duration of 15 months, median progression-free survival (PFS) and overall survival (OS) in SUN/PAZ groups were 8/8 months (p = 0.83) and 22/21 months (p = 0.53), respectively. The common all grade toxicities for SUN vs. PAZ were fatigue (59 vs. 74%), skin changes (44 vs. 44%), anemia (35 vs. 42%), hypothyroidism (37 vs. 19%; p = 0.02) and hypertension (33 vs. 50%). In patients treated with SUN, total grade 3–4 toxicities (mean number of toxic events per patients) were 0.71, whereas in patients treated with PAZ, total grade 3–4 toxicities were 0.11 (p < 0.001). SUN was associated with an increased incidence of grade 3–4 fatigue (p = 0.007), anemia (p = 0.001) and hypothyroidism that needed therapy (p = 0.02). Dose reduction in 49% and 24% of patients (p = 0.02), and treatment cessation in 37% and 26% of patients (p = 0.37) were required in the SUN and PAZ groups, respectively.
ConclusionsIn our study, there was no difference in terms of survival outcomes between two agents. However, patients treated with SUN had more grade 3–4 adverse events which prompted dose reduction.
Sunitinib (SUN) y Pazopanib (PAZ) son dos inhibidores orales de la tirosina kinasa (ITK) que actúan contra el factor de crecimiento endotelial vascular (VEGF, por Vascuar Endothelial Growth Factor). Su eficacia y seguridad en el carcinoma de células renales metastásico (CCRm) se ha demostrado con estudios de fase III. Sin embargo, la evidencia real es escasa. El objetivo de este análisis es evaluar el beneficio clínico de SUN y PAZ en la práctica clínica habitual.
MétodoRevisamos los registros médicos de 79 pacientes con CCRm tratados con SUN (50 mg/día en el régimen 4/2) o PAZ (800 mg/día continuo). Los pacientes fueron evaluados retrospectivamente en dos hospitales turcos entre 2006 y 2016.
ResultadosLa mediana de edad de toda la cohorte fue 60 (28–87) años y el 70% de ellos eran hombres. La tasa de respuesta objetiva (TRO) y la tasa de control de la enfermedad (TCE) en los grupos SUN/PAZ fueron 34/37% (p = 0.96) y 78/87% (p = 0.046), respectivamente. Con una mediana de seguimiento de 15 meses, las medianas de supervivencia libre de progresión (SLP) y de supervivencia general (SG) en los grupos SUN/PAZ fueron de 8/8 meses (p = 0,83) y 22/21 meses (p = 0,53), respectivamente. La toxicidad común entre SUN vs. PAZ incluía fatiga (59 vs. 74%), cambios en la piel (44 vs. 44%), anemia (35 vs. 42%), hipotiroidismo (37 vs. 19%; p = 0.02) e hipertensión (33 vs. 50%). En los pacientes tratados con SUN, la toxicidad total de grado 3–4 (número medio de eventos tóxicos por paciente) fue de 0,71, mientras que, en los pacientes tratados con PAZ, la toxicidad total de grado 3–4 fue de 0,11 (p < 0,001). SUN se asoció con una mayor incidencia de fatiga de grado 3–4 (p = 0.007), anemia (p = 0.001) e hipotiroidismo requiriendo tratamiento (p = 0.02). Fue necesario reducir la dosis en los grupos SUN y PAZ en el 49% y el 24% de los pacientes (p = 0,02), y el cese del tratamiento en el 37% y el 26% de los pacientes (p = 0,37), respectivamente.
Conclusionesen nuestro estudio no hubo diferencias en términos de supervivencia entre los dos agentes. Sin embargo, en los pacientes tratados con SUN se dieron más eventos adversos de grado 3–4, siendo necesaria la reducción de la dosis.
Significant progress has been made in the treatment of mRCC with the advent of targeted therapies against VEGF over the last two decades. SUN is the first approved multi-targeted TKI with antiangiogenic and antitumor activities.1 In Motzer’s trial, it was associated with a higher response rate and longer PFS compared to previous standard treatment, interferon alpha.2 Later, updated results of this study showed a significant improvement in median OS with SUN over interferon alpha (26.4 vs. 21.8 months respectively).3 Other multi-targeted TKI, PAZ also received approval in the first line treatment of mRCC after a pivotal phase III trial that compared PAZ to placebo. In this study, PAZ significantly prolonged the PFS compared to placebo.4 Thus, these two TKIs against VEGF became the standard of care for the first-line treatment in mRCC.
In order to facilitate treatment selection in mRCC, two large randomized trials that compare these two TKIs head to head were designed. The first trial, COMPARZ, was a non-inferiority trial in the first line setting and it revealed that PAZ was not unacceptably worse than SUN in terms of PFS.5 The other trial, PISCES, was a double-blind, cross-over study that took patients’ preferences into consideration. This trial, with its innovative design, demonstrated that PAZ had a better safety profile and significantly more patients preferred PAZ over SUN.6
Despite the results of these randomized clinical trials, physicians still continue to select the medications according to their own experiences and patients’ clinical condition in routine practice. The most important reason is that a more heterogeneous patient population is encountered in everyday practices and they are not represented well enough in clinical trials. Therefore, efficacy and toxicity data are very substantial in these diverse patient populations. There are few real-world data that analyze the efficacy and toxicity of SUN and PAZ retrospectively.7,8 In this study we aimed to review our own findings in mRCC among a wider patient population outside a clinical trial.
Patients and methodsStudy design, patients and proceduresIn this retrospective population-based analysis, we included 79 patients who were treated with either SUN or PAZ in the first line setting for mRCC. They received PAZ as 800 mg per day without interruption or SUN at a dose of 50 mg once daily for 4 weeks followed by a 2-week resting period (4/2 schedule). These patients were a collection of unselected consecutive patient series from two cancer centers in Turkey between 2006 and 2016. The institutional review boards at each participating center approved the study. We collected the demographic baseline patient characteristics and outcome data for targeted therapies with uniform data collection templates. Survival data was obtained retrospectively from medical chart reviews and publicly available records.
Patients were grouped according to Memorial Sloan Kettering Cancer Center (MSKCC) criteria which assessed the five prognostic factors: A serum lactate dehydrogenase concentration of >1.5× the upper limit of the normal range, a hemoglobin concentration below the lower limit, a corrected serum calcium concentration of >10 mg/dl, an interval less than 1 year from initial diagnosis of RCC to treatment, and a Karnofsky performance score of ≤70. Patients were considered as good, intermediate and poor risk if they had no risk factor, 1 or 2 risk factors and 3 or more risk factors, respectively.
To determine the disease response, radiological examination was performed with either a computed tomography or magnetic resonance imaging at baseline and every 3 months after treatment until progression. Response of disease was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1.
Toxicity data were graded according to Common Terminology Criteria (CTC) for Adverse Events, version 4.0. The side effects noted during each visit were collected from patients’ charts. We evaluated these side effects as symptomatic (hand & foot syndrome, mucositis, nausea, fatigue, and diarrhea) and asymptomatic toxicity (raised liver function and hematological toxicity) for each targeted therapy. A proper dose reduction was followed according to the grade of toxic event.
Statistical analysesDescriptive analyses of patients and disease characteristics were performed. Fisher’s exact test was used to compare groups. Comparison between curves was performed using the log-rank test and 95% confidence intervals (CI) for each median time were reported. Response rate (RR) was collected using RECIST 1.1 standards. We assessed overall survival (OS), which was defined as time from first-line targeted therapy initiation to death or censored at last follow-up. PFS was defined as time from first-line targeted therapy initiation to progression, death, treatment cessation, or censored at last follow-up. We performed summary statistics and estimated censored outcomes using Kaplan-Meier curves.
All p values were two-sided, with p < 0.05 indicating statistical significance. All statistical analyses were performed using the SPSS package (version 20.0).
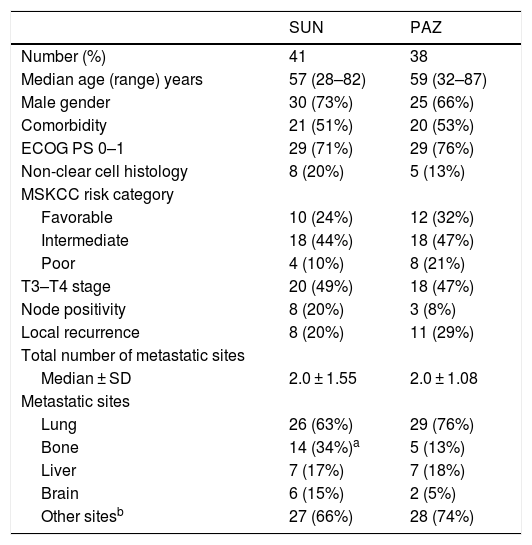
ResultsPatient characteristics (Table 1)We obtained data from 79 patients with mRCC treated with either first-line SUN (n = 41) or PAZ (n = 38). For entire cohort median age of patients was 60 (28–87) years and 70% of them were male. Diabetes mellitus, hypertension, coronary artery disease, and chronic renal insufficiency were evaluated as comorbid diseases. Fifty two percent of patients have co-morbidities accompanying mRCC. Eastern Cooperative Oncology Group (ECOG) performance status was 0 and 1 in 73% of patients. Biopsy was done in 22% of patients for diagnosis. Thirteen patients (17%) had non-clear cell carcinoma histology. The baseline patient and tumor characteristics were summarized in Table 1 according to individual treatment group. In SUN group, 4 patients had chromophobe, and other 4 patients had papillary histology. In PAZ group, 3 patients with papillary, 1 patient with sarcomatoid and 1 patient with succinate dehydrogenase deficiency were diagnosed as non-clear cell histology. There was no statistically significant difference in terms of patient and tumor characteristics between two TKIs except bone metastases. In patients treated with SUN, bone metastases were seen in 34% of patients. However, in PAZ group 13% of patients had bone metastases (p = 0.03).
Patient characteristics.
| SUN | PAZ | |
|---|---|---|
| Number (%) | 41 | 38 |
| Median age (range) years | 57 (28–82) | 59 (32–87) |
| Male gender | 30 (73%) | 25 (66%) |
| Comorbidity | 21 (51%) | 20 (53%) |
| ECOG PS 0–1 | 29 (71%) | 29 (76%) |
| Non-clear cell histology | 8 (20%) | 5 (13%) |
| MSKCC risk category | ||
| Favorable | 10 (24%) | 12 (32%) |
| Intermediate | 18 (44%) | 18 (47%) |
| Poor | 4 (10%) | 8 (21%) |
| T3–T4 stage | 20 (49%) | 18 (47%) |
| Node positivity | 8 (20%) | 3 (8%) |
| Local recurrence | 8 (20%) | 11 (29%) |
| Total number of metastatic sites | ||
| Median ± SD | 2.0 ± 1.55 | 2.0 ± 1.08 |
| Metastatic sites | ||
| Lung | 26 (63%) | 29 (76%) |
| Bone | 14 (34%)a | 5 (13%) |
| Liver | 7 (17%) | 7 (18%) |
| Brain | 6 (15%) | 2 (5%) |
| Other sitesb | 27 (66%) | 28 (74%) |
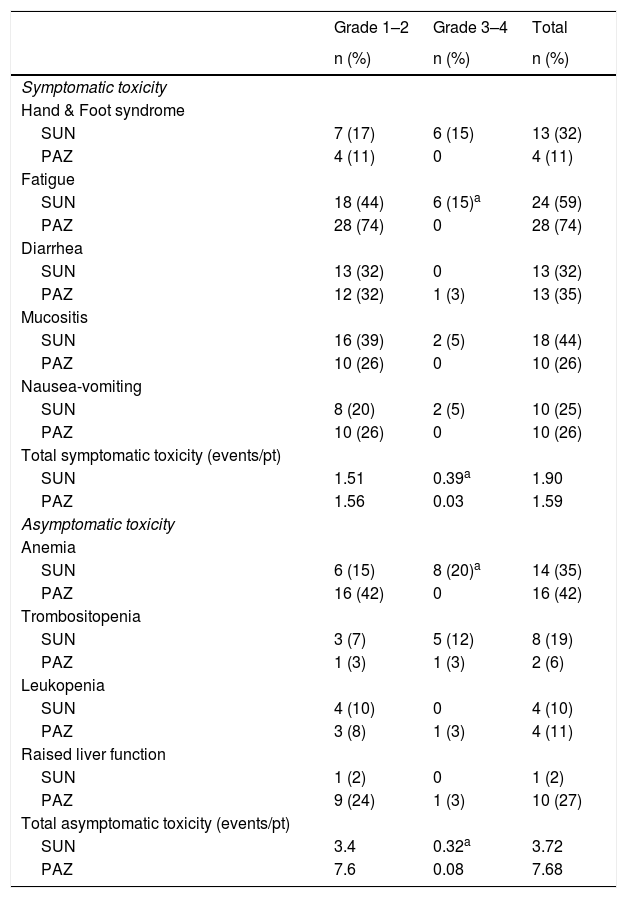
In the SUN group, the most common non-hematologic toxicities were fatigue (59%), skin changes (44%), and hypothyroidism (37%); while anemia (35%) was the most common hematologic toxicity. In the PAZ group, the most common non-hematologic toxicities were fatigue (74%), hypertension (50%), and skin changes (44%); while anemia (42%) was the most common hematologic toxicity. The other toxicities were pretibial edema, genital wounds, acute renal failure, hematuria, epistaxis, and gastrointestinal bleeding.
Comparison of toxicity observed.
| Grade 1–2 | Grade 3–4 | Total | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Symptomatic toxicity | |||
| Hand & Foot syndrome | |||
| SUN | 7 (17) | 6 (15) | 13 (32) |
| PAZ | 4 (11) | 0 | 4 (11) |
| Fatigue | |||
| SUN | 18 (44) | 6 (15)a | 24 (59) |
| PAZ | 28 (74) | 0 | 28 (74) |
| Diarrhea | |||
| SUN | 13 (32) | 0 | 13 (32) |
| PAZ | 12 (32) | 1 (3) | 13 (35) |
| Mucositis | |||
| SUN | 16 (39) | 2 (5) | 18 (44) |
| PAZ | 10 (26) | 0 | 10 (26) |
| Nausea-vomiting | |||
| SUN | 8 (20) | 2 (5) | 10 (25) |
| PAZ | 10 (26) | 0 | 10 (26) |
| Total symptomatic toxicity (events/pt) | |||
| SUN | 1.51 | 0.39a | 1.90 |
| PAZ | 1.56 | 0.03 | 1.59 |
| Asymptomatic toxicity | |||
| Anemia | |||
| SUN | 6 (15) | 8 (20)a | 14 (35) |
| PAZ | 16 (42) | 0 | 16 (42) |
| Trombositopenia | |||
| SUN | 3 (7) | 5 (12) | 8 (19) |
| PAZ | 1 (3) | 1 (3) | 2 (6) |
| Leukopenia | |||
| SUN | 4 (10) | 0 | 4 (10) |
| PAZ | 3 (8) | 1 (3) | 4 (11) |
| Raised liver function | |||
| SUN | 1 (2) | 0 | 1 (2) |
| PAZ | 9 (24) | 1 (3) | 10 (27) |
| Total asymptomatic toxicity (events/pt) | |||
| SUN | 3.4 | 0.32a | 3.72 |
| PAZ | 7.6 | 0.08 | 7.68 |
Dose reductions and cessation during therapy.
| SUN | PAZ | |
|---|---|---|
| n (%) | n (%) | |
| Dose reduction | 20 (49%)a | 9 (24%) |
| Treatment cessation | 15 (37%) | 10 (26%) |
There was no significant difference in the overall number of toxic events (mean number of toxic events/patients 5.62% vs. 9.27%) and the occurrence of symptomatic toxicity for SUN and PAZ (mean number of toxic events/patients 1.90 vs. 1.59; p > 0.05). However, SUN was associated with an increased incidence of grade 3–4 symptomatic toxicity (mean number of symptomatic toxic events/patients 0.39 vs. 0.03; p < 0.001). Specifically, fatigue was significantly increased in SUN group (p = 0.007). Incidence of grade 3–4 asymptomatic toxicity was also increased in SUN (mean number of toxic events/patients 0.32 vs. 0.08; p < 0.001). A grade 3–4 anemia was significantly higher in SUN group (p = 0.001). In all patients who received SUN, total (asymptomatic and symptomatic toxicity) grade 3–4 toxicities (mean number of toxic events/patients) were 0.71. However, in patients treated with PAZ, total grade 3–4 toxicities were 0.11 (p < 0.001) (Table 2). Hypothyroidism that required treatment was significantly higher in SUN compared to PAZ group (37 vs. 19%; p = 0.02). There was no significant difference in hypertension for SUN and PAZ groups, respectively (33 vs. 50%; p = 0.48). Dose reduction was performed in 50% of patients in SUN and 24% of patients in PAZ (p = 0.02). Also 37% of patients in SUN and 26% of patients in PAZ group discontinued the treatment due to side effects (p = 0.37) (Table 3).
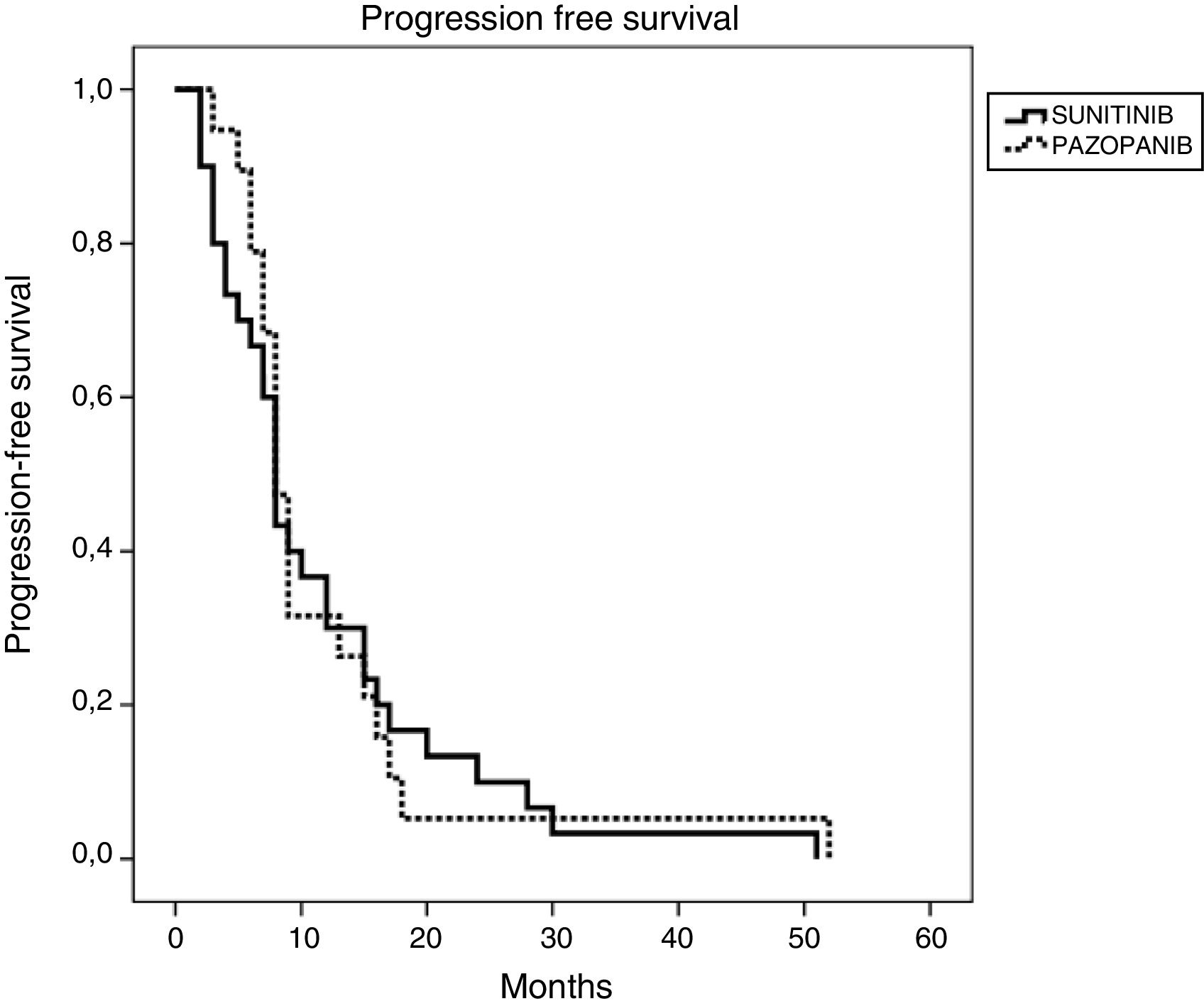
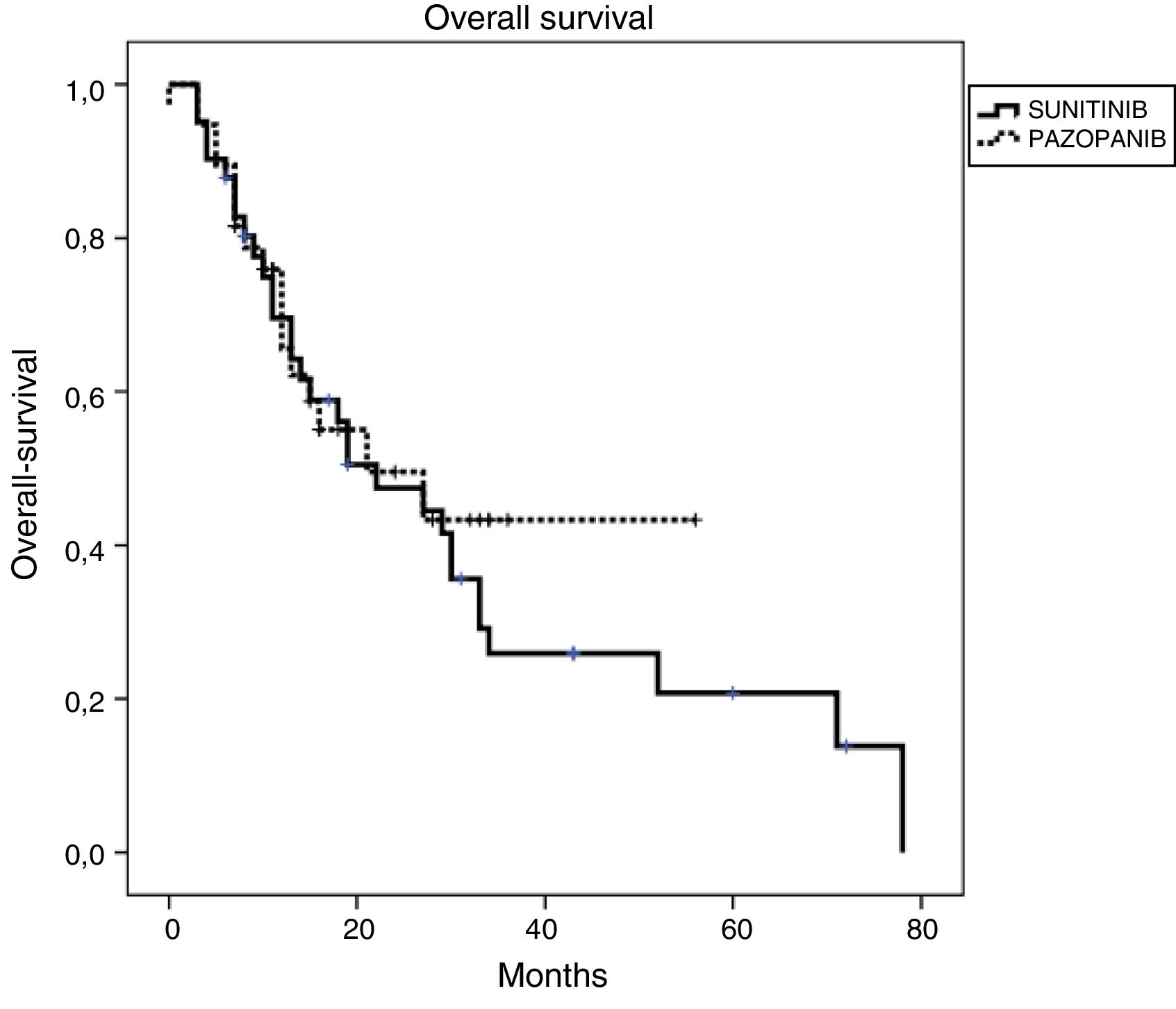
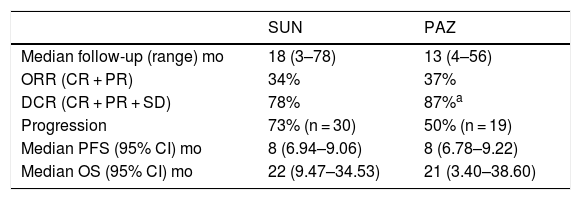
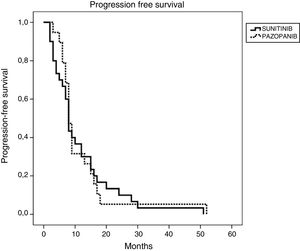
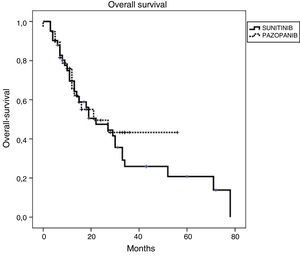
Efficacy and survival outcomes (Table 4, Figs. 1 and 2)Median follow-up time was 18 months and 13 months for SUN and PAZ groups, respectively (p = 0.21). In SUN group, 73% of patients were identified as having tumor progression and 73% of them died whereas in PAZ group, 50% of patients were identified as having tumor progression, and 45% of them died (p = 0.08). Overall response rate was 34% and 37% (p = 0.96) and disease control rate was 78% and 87% (p = 0.046) for SUN and PAZ groups, respectively. Median PFS was 8 months (95% CI: 6.9–9.1) in patients treated with SUN, and 8 months (95% CI: 6.8–9.2) in patients treated with PAZ (p = 0.83). Median OS was 22 (95% CI: 9.5–34.5) months in SUN group, and 21 (95% CI: 3.4–38.6) months in PAZ group (p = 0.53).
Efficacy and survival outcomes.
| SUN | PAZ | |
|---|---|---|
| Median follow-up (range) mo | 18 (3–78) | 13 (4–56) |
| ORR (CR + PR) | 34% | 37% |
| DCR (CR + PR + SD) | 78% | 87%a |
| Progression | 73% (n = 30) | 50% (n = 19) |
| Median PFS (95% CI) mo | 8 (6.94–9.06) | 8 (6.78–9.22) |
| Median OS (95% CI) mo | 22 (9.47–34.53) | 21 (3.40–38.60) |
CR: Complete Response, PR: Partial Response, SD: Stable Disease, ORR: Overall Response Rate, DCR: Disease Control Rate.
This is the first real-world efficacy and toxicity data of our mRCC patients who received either SUN or PAZ in Turkey. Patients on PAZ had median PFS of 8 months and median OS of 21 months, whereas patients on SUN had median PFS of 8 months and median OS of 22 months. In SUN arm, there were more patients with bone metastases compared with PAZ arm, but it did not impact survival analyses. There was no difference in terms of median PFS and median OS between two medication groups.
Our OS outcomes were less when compared with survivals in COMPARZ trial. In this trial survival analyses, interpreted by an independent review, showed median PFS/OS of 8.4/28.4 months for PAZ and 9.5/29.3 months for SUN, respectively.5 Nevertheless, it is very difficult to compare these two trials especially when patients’ characteristics were considered. In our study, patients were not selected as in clinical trials. Twenty percent of them had brain metastases and 17% of them had non-clear cell histology. Patients with poor performance score were also included into this study. Moreover, half of the patients had comorbid diseases that accompany mRCC. All these unfavorable features could compromise our OS outcomes, but both medications controlled the disease in similar periods as in COMPARZ trial.
Recently two large retrospective real-world survival data, which compared SUN with PAZ in mRCC, had been published. The first trial, international metastatic renal cell carcinoma database consortium (IMDC) which is a retrospective data of unselected patients collected from 29 different cancer centers reported similar median OS (22.3 vs. 22.6 months) and median PFS (8.4 vs. 8.3 months) for SUN and PAZ, respectively. The percentage of non-clear cell histology was almost half (10/7% vs. 20/13%) of our study. Also, the percentage of patients with KPS less than 80 was reported as 13/14%, whereas ECOG PS 2 and 3 was 29/24% in our SUN/PAZ groups.7 Despite these differences, survival outcomes reported were comparable to our study results. The other retrospective data was from Canada published by Dr. Lalani and colleges. In this study investigators showed that patients treated with SUN had improved OS compared with PAZ (median 31.7 vs. 20.6 months; p = 0.028). In their cohort, patients treated with SUN were prone to be younger with good performance score than PAZ group. They stated that improvement of OS might be related with patient selection and using different SUN schedules in their common practice.8 Actually, there are studies that introduce alternative schedules to keep patients as much as possible on treatment.9–11 These altered schedules could promote favorable prognosis by decreasing adverse events caused by SUN. Recently in our clinical practices we began to offer different SUN schedules or switch to alternative schedules, but in this study only dose reduction was performed according to the grade of toxic event.
As the efficacy results of these two TKIs are similar, safety profile has become even more important in routine clinical practice. Side effects of these drugs are quite different than the chemotherapy side effect profile that we are used to. In case of SUN and PAZ, emerging side effects during therapy does not relieve as these drugs are taken continuously. And this situation affects negatively both quality of life and compliance of patients. Therefore, it is crucial to distinguish the symptomatic and asymptomatic toxicities and manage them accordingly. In our work, we observe more hand and foot syndrome, mucositis, thrombocytopenia and hypothyroidism with SUN and increased liver function tests with PAZ. There was no difference in overall number of toxic events between SUN and PAZ, but when these results were studied in detail, we realized that grade 3–4 symptomatic and asymptomatic toxicities were significantly higher with SUN. Specifically, grade 3–4 anemia, grade 3–4 fatigue and hypothyroidism were more prevalent in SUN group.
In COMPARZ trial grade 3–4 side effects that will disturb patients’ comfort like fatigue, hand foot syndrome and mucositis were found more frequently with SUN. Other grade 3–4 laboratory abnormalities such as anemia, thrombocytopenia and neutropenia had been increased significantly in SUN group. There were also more patients with hypothyroidism on SUN group but the difference between two medications was not significant. Among patients on PAZ, researchers detected significantly increased grade 3–4 liver function tests.5 Recently another trial that looked the safety profile of Asian and non-Asian subpopulations by using the data from COMPARZ was published. In this study, increased incidences of hypertension, hematologic toxicities, abnormal liver and renal function tests and hand and foot syndrome were found in Asian patients. Conversely, in non-Asian patients’ gastrointestinal side effects, headache and dyspnea were observed with TKIs. Among Asian patients, SUN treated group experienced more hematologic and non-hematological toxicities than PAZ treated group.12 Powles and colleagues also reported more severe symptomatic toxicity as fatigue and mucositis in SUN group.13 Similar results were observed in our study in Turkey, which is located in a transition region between Europe and Asia. But on the contrary of previous studies, our patients with increased liver function tests on PAZ were often evaluated as grade 1–2 toxicity, which is consistent with the results of another study from South Korea.14 Researchers from Canada observed less fatigue with SUN and explained this result with altered SUN schedule.8
In our trial, patients on SUN had significantly more dose reduction (49%) when compared to PAZ (24%). However, dose reduction was similar for SUN (51%) and PAZ (44%) in COMPARZ trial.5 Also, in another report that analyses safety of SUN and PAZ in Asian and non-Asian subpopulations, researches reported slightly higher dose reduction or interruption in Asian population compared with non-Asian population. But, the rates of dose reduction for SUN and PAZ were similar in both Asian and non-Asian populations.12 Also, discontinuation rates due to adverse events were reported relatively high among non-Asian patient population and Canadian patients on PAZ.8,12 Nevertheless, discontinuation of SUN due to adverse events was observed slightly more in our study as in Powles’s report. These different toxicities observed in different ethnic groups can be due to the genetic polymorphism on TKIs’ pharmacokinetic and pharmacodynamics.15 Realizing these side effects in different patient population is particularly important. As it is understood from studies that there is an urgent need for proper management of these side effects for favorable prognosis. Literature indicates that there is a positive correlation with plasma concentration of TKI and outcomes.16
There are several shortcomings of this study due to its retrospective nature. Small sample size and missing data on MSKCC scoring in SUN group should be interpreted cautiously. Since physicians tend to focus more on the side effects that impair patient comfort during the visits, a comprehensive documentation of adverse effects has been limited in our charts. Some adverse events like altered taste is underreported. Despite these limitations, patients were included in this study in a consecutive way. Sample size in both treatment groups was fairly balanced. Patient population was heterogeneous in both treatment groups reflecting our general clinic. And we were able to abstract most of side effects from the charts that could guide our treatment judgment in real world practice.
In conclusion, SUN and PAZ had similar efficacy in terms of survival outcomes, but side effect profile favored PAZ in our unselected heterogeneous patient population with mRCC. Nonetheless, this data is not sufficient enough to make a specific recommendation about the accurate selection of first line therapy in mRCC. This is because altered schedule of SUN or individualized dosing of TKIs has not been examined in this current study.
DisclosureAll authors have declared no conflicts of interest.
FundingNone.
Please cite this article as: Ekenel M, Karabulut S, Cil I, Zırtıloglu A, Aydın E, Tural D. Sunitinib versus pazopanib para el tratamiento del carcinoma de células renales metastásico: experiencia en 2 hospitales turcos. Actas Urol Esp. 2019. https://doi.org/10.1016/j.acuro.2019.06.007