DD3PCA3 (PCA3) gene expression is prostate cancer-specific. Routine use of this biomarker has resulted in a 35–67% reduction in the number of required biopsies. The aim of this study is to evaluate our outcomes in its routine use and to establish in which group of patients this is the most efficient, depending on the number of previous PCA3 biopsies.
Material and methodsA total of 474 consecutive patients who had previously undergone a biopsy (group A, n=337) or not (group B, n=134) for whom a PCA3 was requested were analyzed. We subdivided group A into A1 (a previous biopsy, n=182) and A2 (<1 previous biopsy, n=155). The recommendation of whether to perform a biopsy or not was made independently by each of the 11 clinicians and guided by prostatic specific antigen (PSA) levels and digital rectal examination.
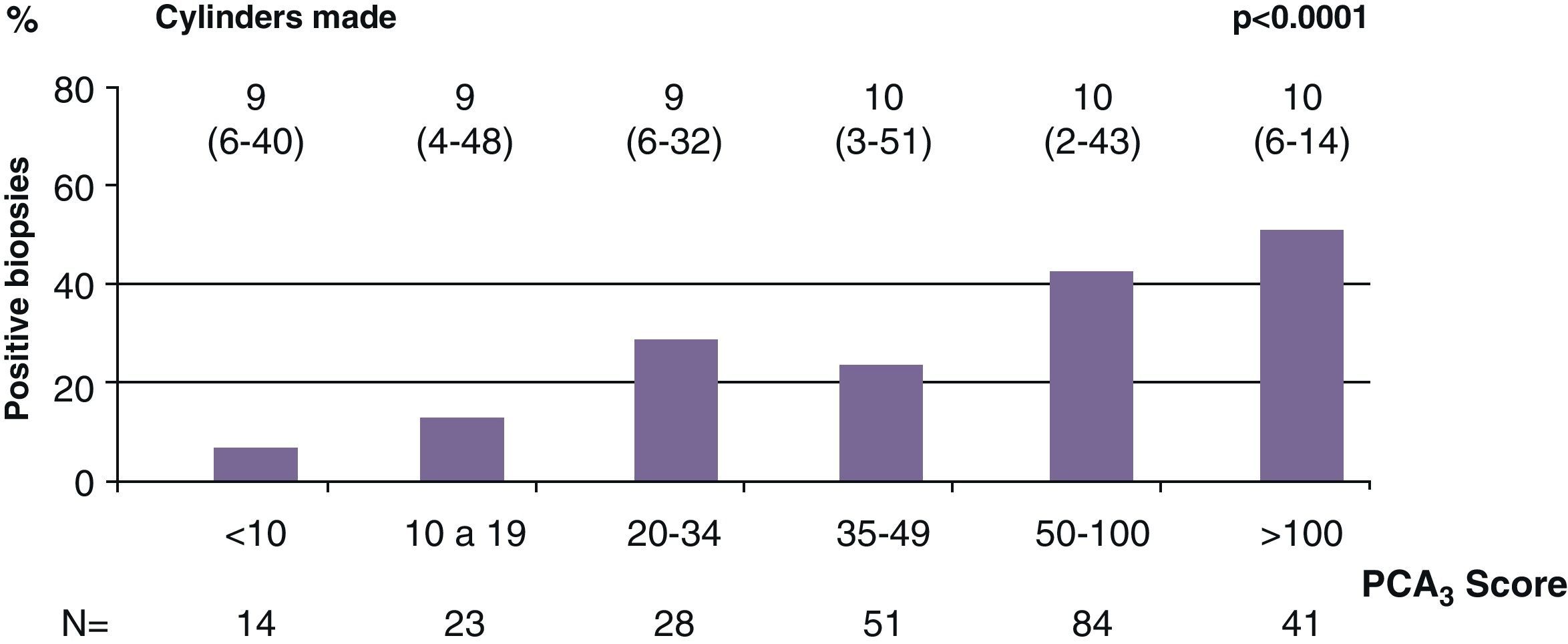
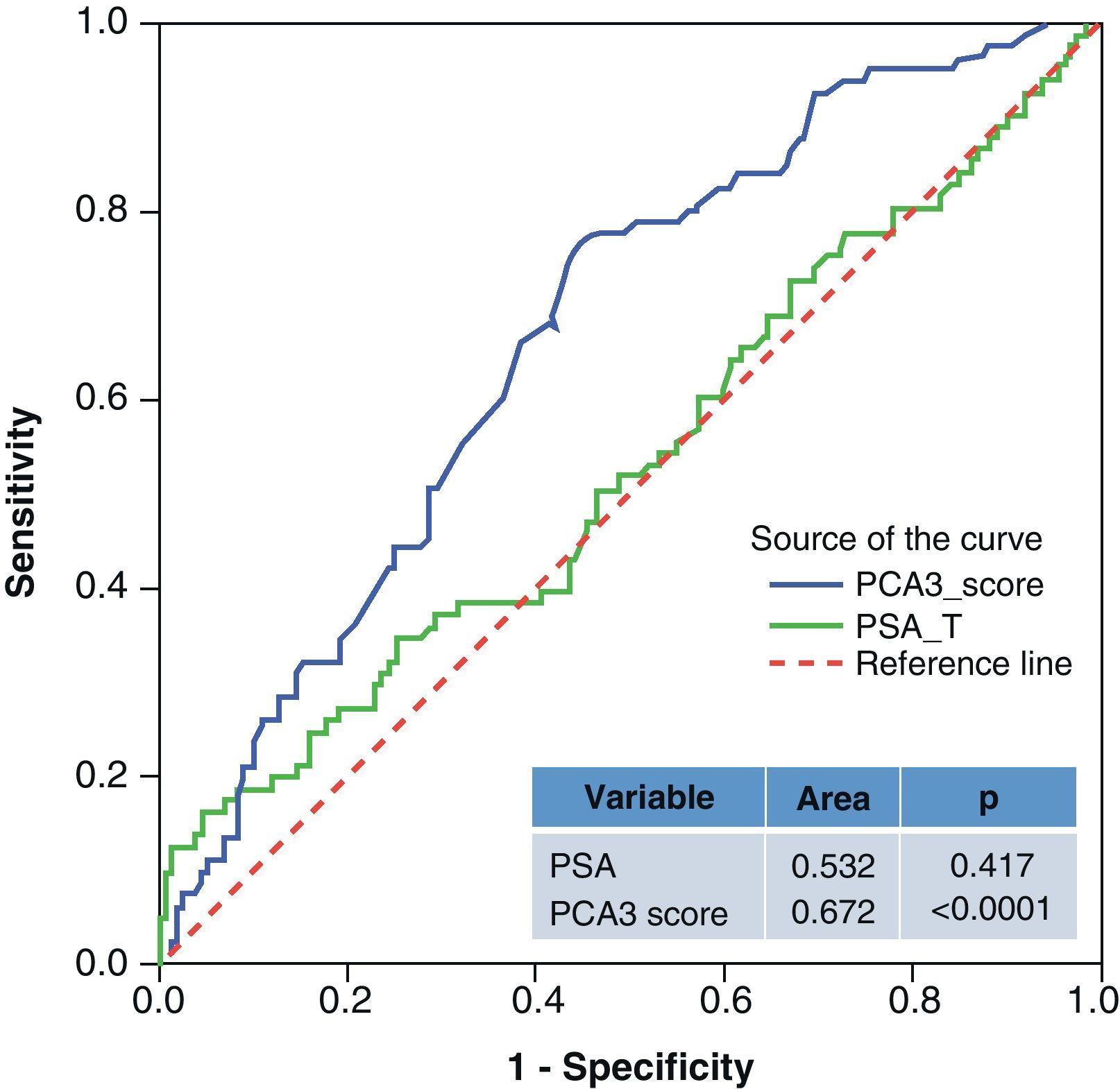
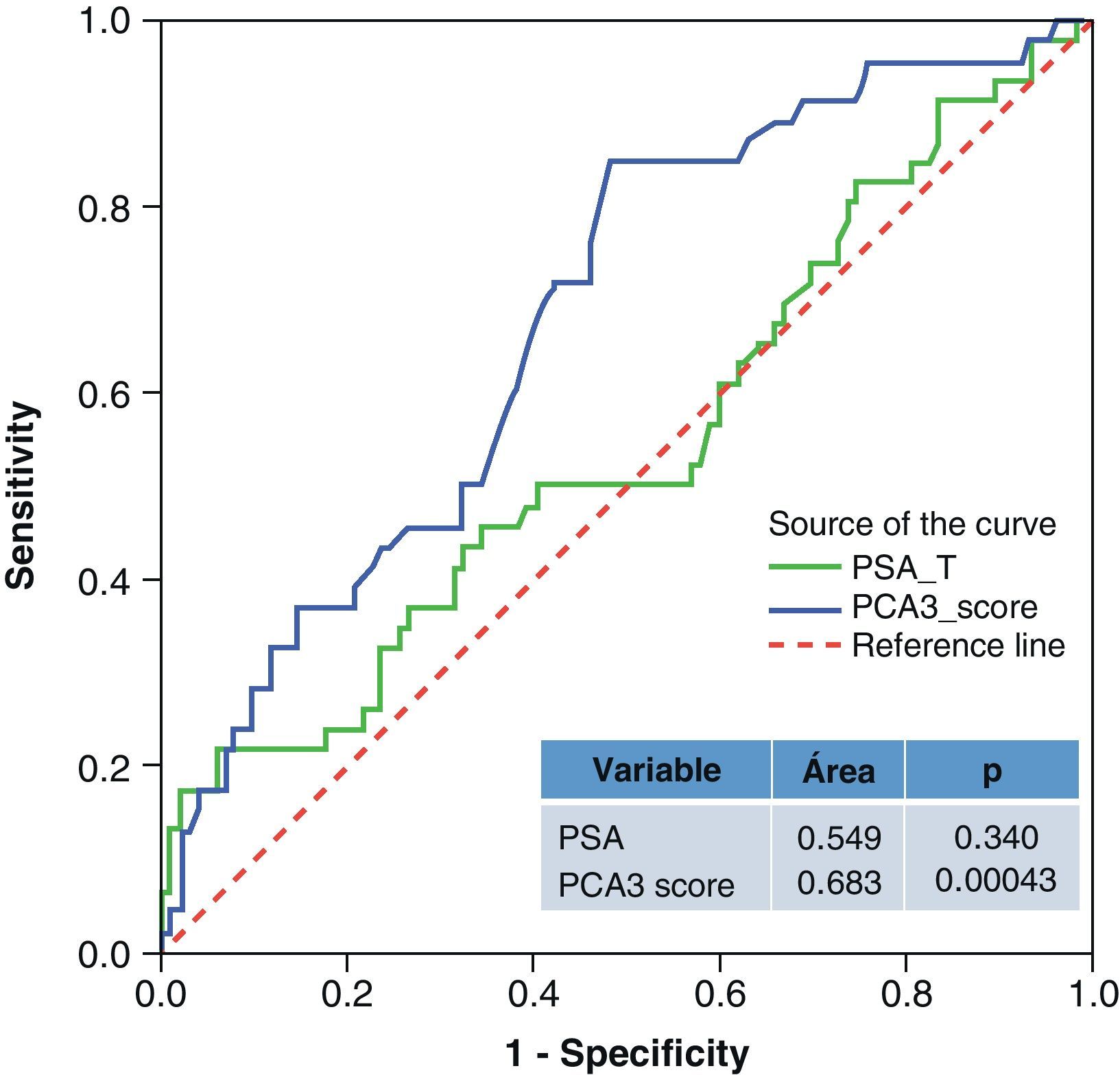
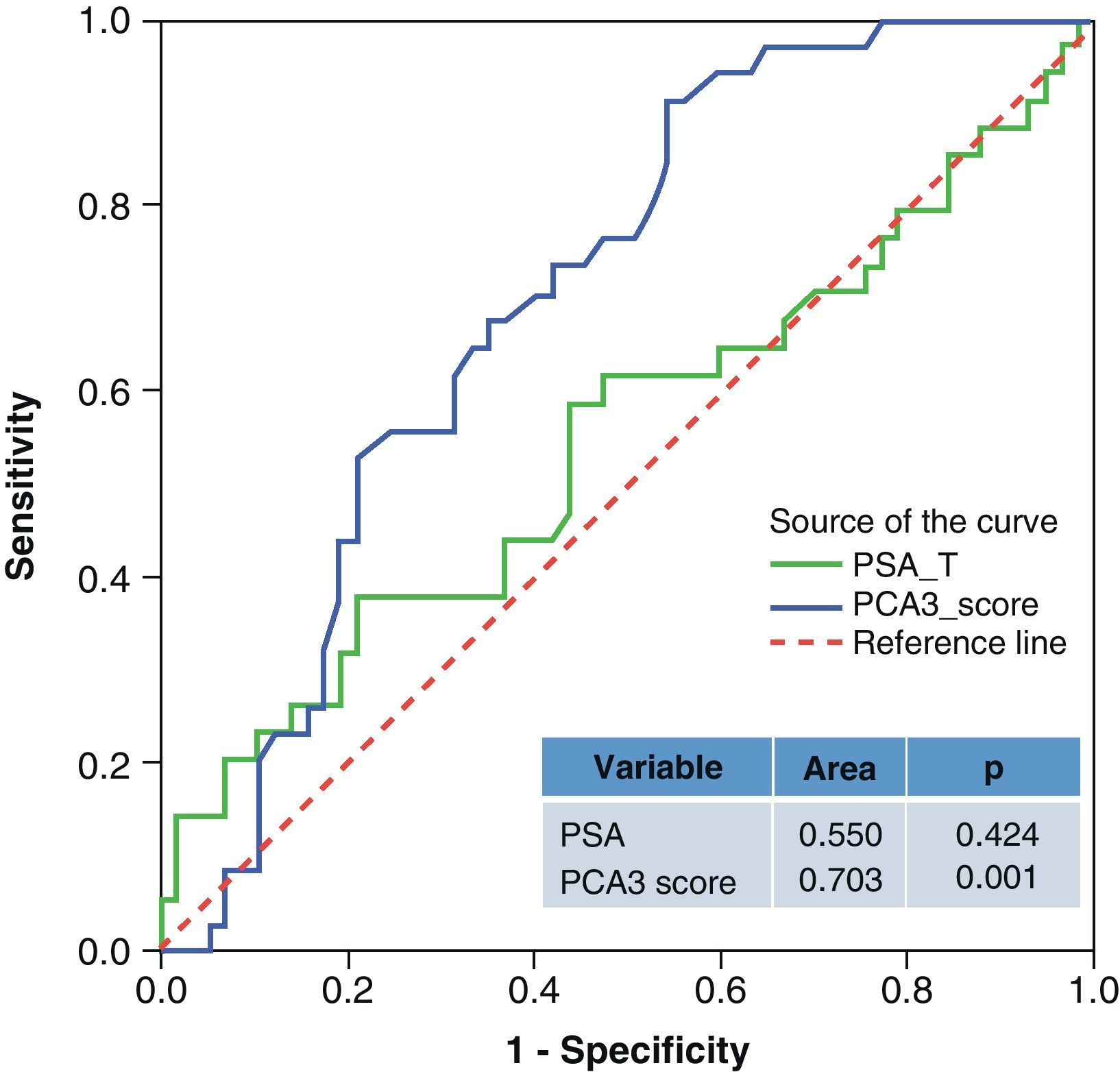
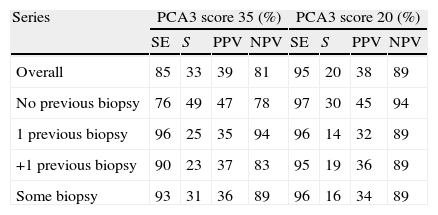
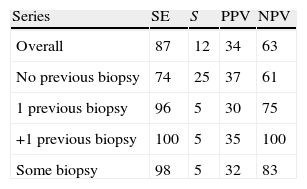
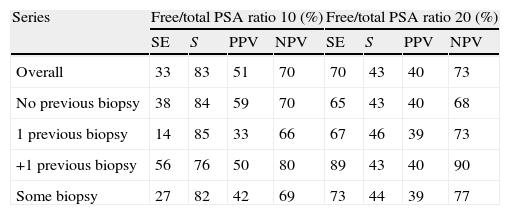
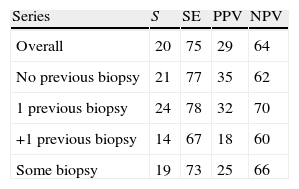
ResultsMedian age was 65 years (range 38–84). PCA3 score had an informative ratio of 99.6%, with a median of 29 (range 1–3245). The percentage of biopsy sparing was 49% of the cases. ROC analysis demonstrated an AUC for PSA and PCA3 of 0.532 (p=0.417) and 0.672 (p<0.0001), respectively. Sensitivities of PSA ≥4 and PCA3 ≥35 were 87% vs. 85%, with specificities of 12% vs. 33%, PPV 34% vs. 39% and NPV 63% vs. 81%, respectively. The PCA3 score showed direct correlation with the percentage of positive biopsies (p<0.0001).
ConclusionsRoutine use of PCA3, due to its high NPV, results in a significant reduction in the number of biopsies. PCA3 appears to be more efficient in biopsy-naive patients. Among patients already biopsied, the results are superior in those biopsied only once.
La expresión del gen DD3PCA3 (PCA3) es específica del cáncer de próstata. El porcentaje de biopsias que se pueden ahorrar con este biomarcador es de 35–67%. Nuestro objetivo es analizar los resultados en uso rutinario y establecer en qué subgrupo de pacientes es más rentable según el número de biopsias previas.
Material y métodosAnalizamos a 474 pacientes, biopsiados previamente (grupo A, n=337) o no (grupo B, n=134) en los que se solicitó el PCA3. Subdividimos el grupo A en A1 (una biopsia previa, n=182) y A2 (>1 biopsia previa, n=155). La recomendación de biopsiar o no se tomó de forma independiente por cada uno de los urólogos del Servicio junto con el antígeno prostático específico (PSA) y tacto rectal.
ResultadosLa mediana de edad fue 65 años (rango 38–84). La tasa informativa del PCA3 score fue del 99,6% y su mediana 29 (rango 1–3245). El porcentaje de ahorro de biopsias fue 49%. Las áreas bajo la curva ROC para PSA y PCA3 fueron de 0,532 (p=0,417) y 0,672 (p<0,0001). La sensibilidad de PSA ≥4 y PCA3 ≥35 fueron 87 y 85%, la especificidad 12 y 33%, el valor predictivo positivo (VPP) 34 y 39% y el valor predictivo negativo (VPN) 63 y 81%. Tomado el valor de PCA3 como variable contínua, a mayor PCA3 obtenemos mayor porcentaje de biopsias positivas (p<0,0001).
ConclusionesEl uso rutinario del PCA3 ahorra la mitad de las biopsias, basándose sobre todo en su alto VPN. La mayor rentabilidad diagnóstica del PCA3 la obtenemos en pacientes sin biopsia. Entre los pacientes ya biopsiados, los resultados son ligeramente mejores en aquellos con solo una.