High-dose rate brachytherapy (HDR-BT) is an increasingly popular treatment for patients with localized prostate cancer (PC).
ObjectiveTo assess the safety and efficacy of HDR-BT as monotherapy in PC.
Acquisition of evidenceA systematic literature review was conducted through searches on MEDLINE (PubMed), Cochrane Library, CDR, ClinicalTrials and EuroScan. We assessed safety and efficacy indicators.
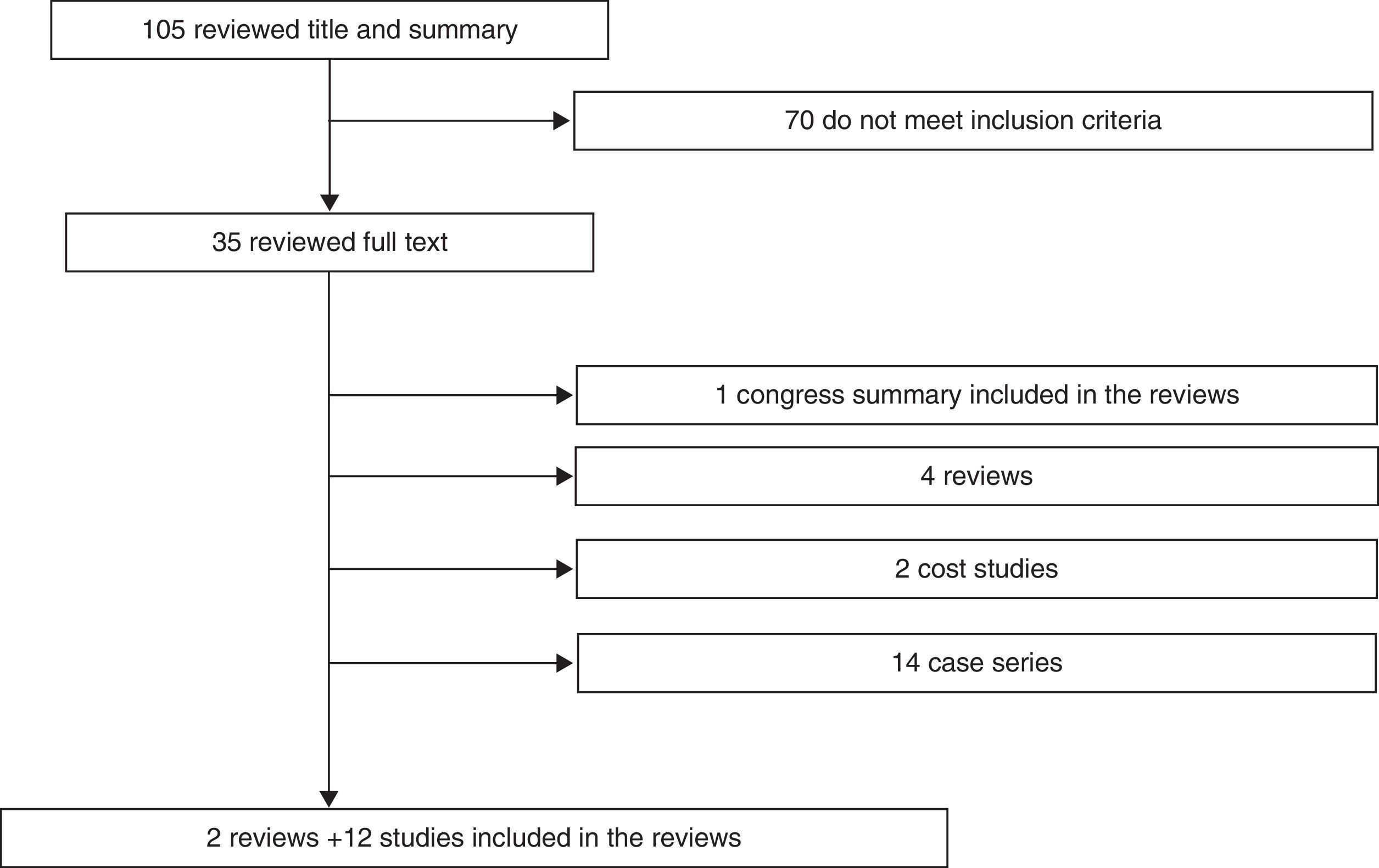
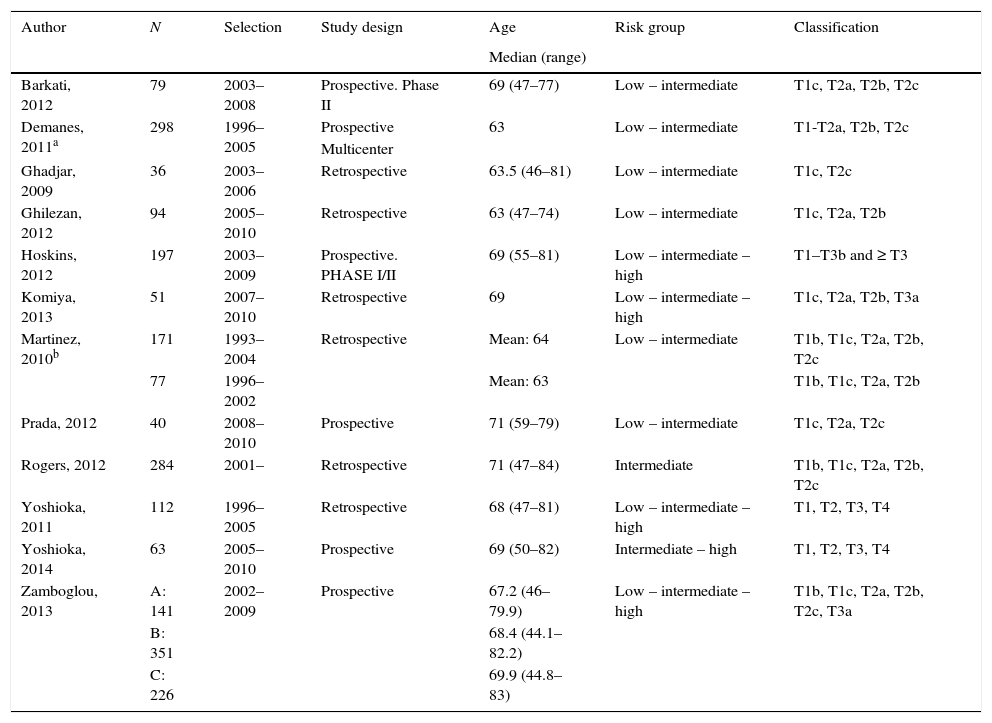
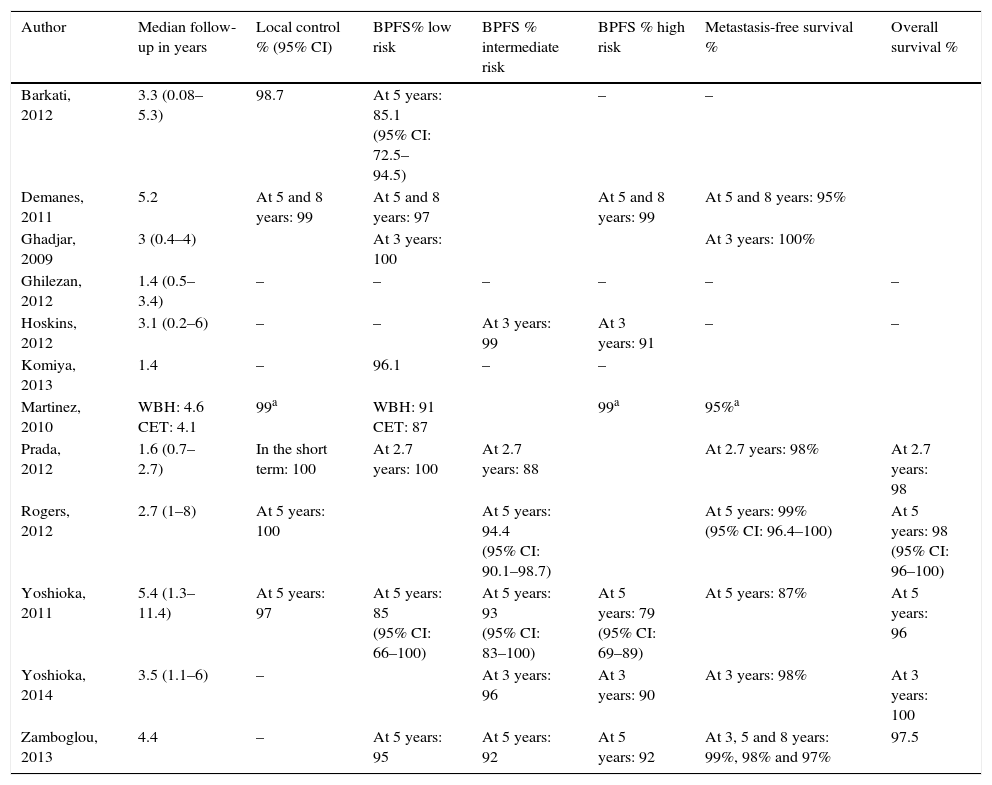
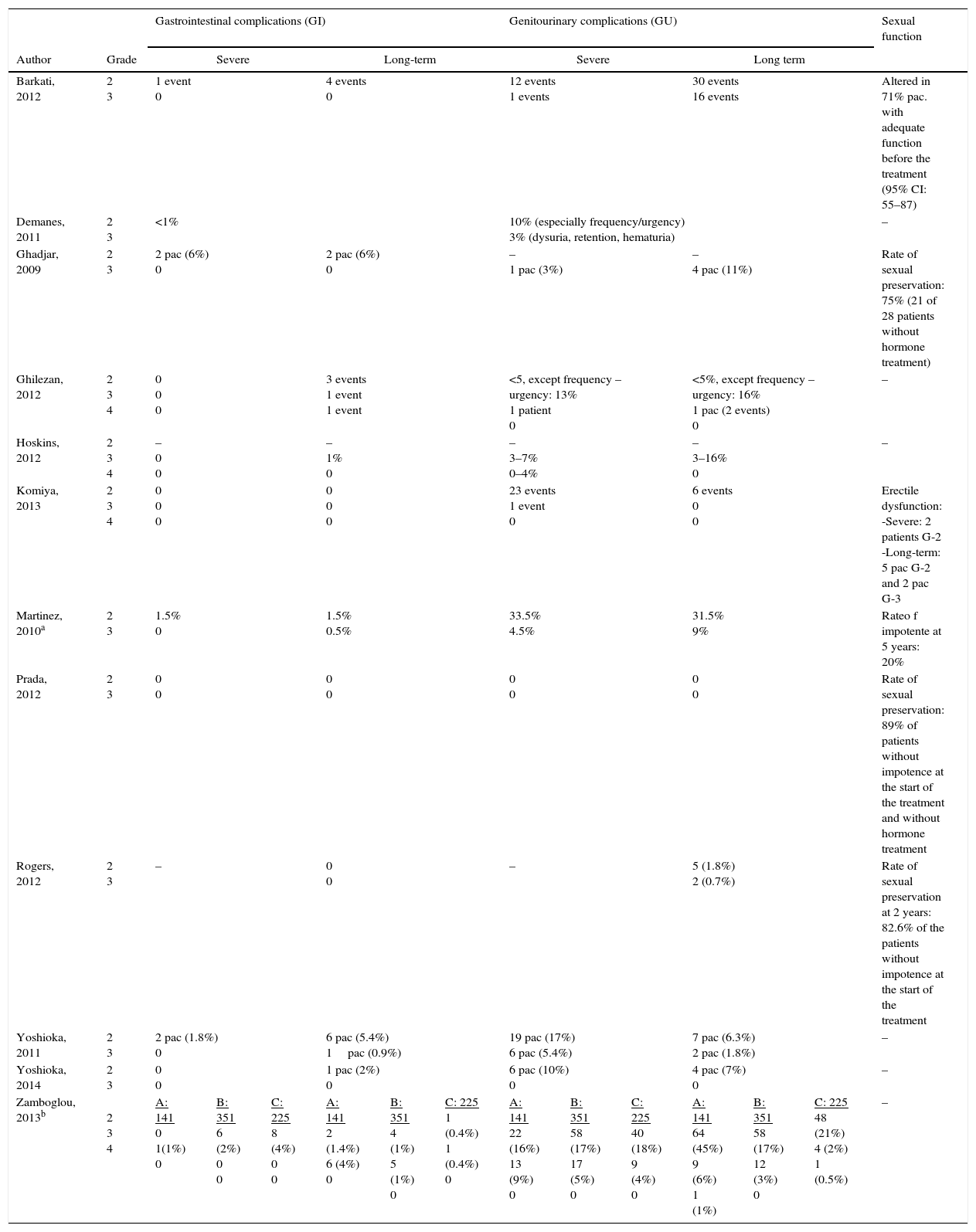
Summary of the evidenceWe selected 2 reviews and 12 uncontrolled studies, included in these 2 reviews. In terms of efficacy, local control in 6 studies was 97–100%. The biochemical progression-free survival varied as follows: 85–100% for low risk and 79–92% for high risk. Survival free of metastases was >95% at 8 years, except in one study where the survival rate was 87% at 5 years. The overall survival was ≥95% in 8 studies. In terms of safety, most of the studies recorded acute and long-term genitourinary and gastrointestinal complications, especially grade ≥2. Only 3 studies found grade 4 complications. All studies, except for one without complications, observed genitourinary complications that were more frequent and severe than the gastrointestinal complications. Two studies assessed the quality of life and showed an initial reduction in various domains and subsequent partial or total recovery, except in the sexual domain.
ConclusionsHDR-BT is effective as monotherapy, especially in cases of low to intermediate risk. There is insufficient information on high-risk patients. The short to medium-term toxicity was acceptable. Further research needs to be funded to provide more information on the long-term safety and efficacy of this treatment.
La braquiterapia con alta tasa de dosis (HDR-BT) es un tratamiento de uso creciente en pacientes con cáncer de próstata (CP) localizado.
ObjetivoEvaluar la eficacia y seguridad de HDR-BT como monoterapia en CP.
Adquisición de evidenciaRevisión sistemática de la literatura mediante búsqueda en MEDLINE (PubMed), Cochrane Library, CDR, Clinicaltrials y EuroScan. Se evaluaron indicadores de eficacia y de seguridad.
Síntesis de evidenciaFueron seleccionadas 2 revisiones y 12 estudios no controlados, incluidos en estas 2 revisiones. En términos de eficacia, el control local en 6 estudios es 97-100%. La supervivencia libre de progresión bioquímica varía: 85-100% en riesgo bajo y 79-92% en riesgo alto. La supervivencia libre de metástasis es >95% a 8 años, salvo en un estudio que es 87% a 5 años. La supervivencia global es ≥95% en 8 estudios. En relación con la seguridad, la mayoría de los estudios recogen complicaciones genitourinarias y gastrointestinales agudas y a largo plazo, especialmente de grado ≥2. Solo 3 estudios encuentran complicaciones grado 4. Excepto uno (sin complicaciones), en los 11 restantes las complicaciones complicaciones genitourinarias son más frecuentes y más graves que las gastrointestinales. Dos estudios evalúan la calidad de vida y muestran un descenso inicial en distintos dominios y posterior recuperación parcial o total, salvo en la esfera sexual.
ConclusionesLa HDR-RT como monoterapia es eficaz, especialmente en riesgo bajo e intermedio. No existe suficiente información en pacientes de riesgo alto. La toxicidad a corto-medio plazo es aceptable. Consideramos necesario potenciar la investigación que aporte más información sobre eficacia y seguridad a largo plazo de este tratamiento.