Prostate photovaporisation with Greenlight laser for the surgical treatment of benign prostate hyperplasia has rapidly evolve to the new XPS 180W.
We have previously demonstrated the safety and efficacy of the HPS 120W. The aim of this study was to assess the functional and safety results, with a year of follow-up, of photovaporisation using the XPS 180W laser compared with its predecessor.
Material and methodsA cohort study was conducted with a series of 191 consecutive patients who underwent photovaporisation between 1/2008 and 5/2013.
The inclusion criteria were an international prostate symptom score (IPSS) >15 after medical failure, a prostate volume <80cm3 and a maximum flow <15ml/s.
We assessed preoperative and intraoperative variables (energy used, laser time and total surgical time), complications, catheter hours, length of stay and functional results (maximum flow, IPSS, prostate-specific antigen and prostate volume) at 3, 6 and 12 months.
We analyzed the homogeneity in preoperative characteristics of the 2 groups through univariate analysis techniques. The postoperative functional results were assessed through an analysis of variance of repeated measures with mixed models.
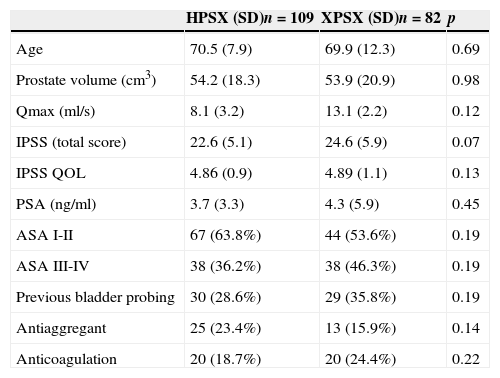
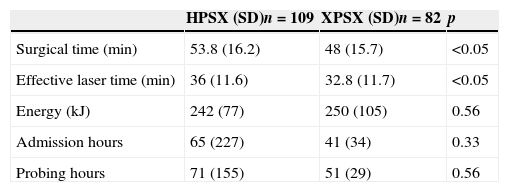
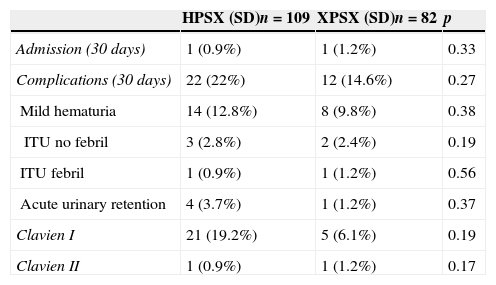
ResultsA total of 109 (57.1%) procedures were performed using HPS 120W, and 82 (42.9%) were performed using XPS. There were no differences between the preoperative characteristics.
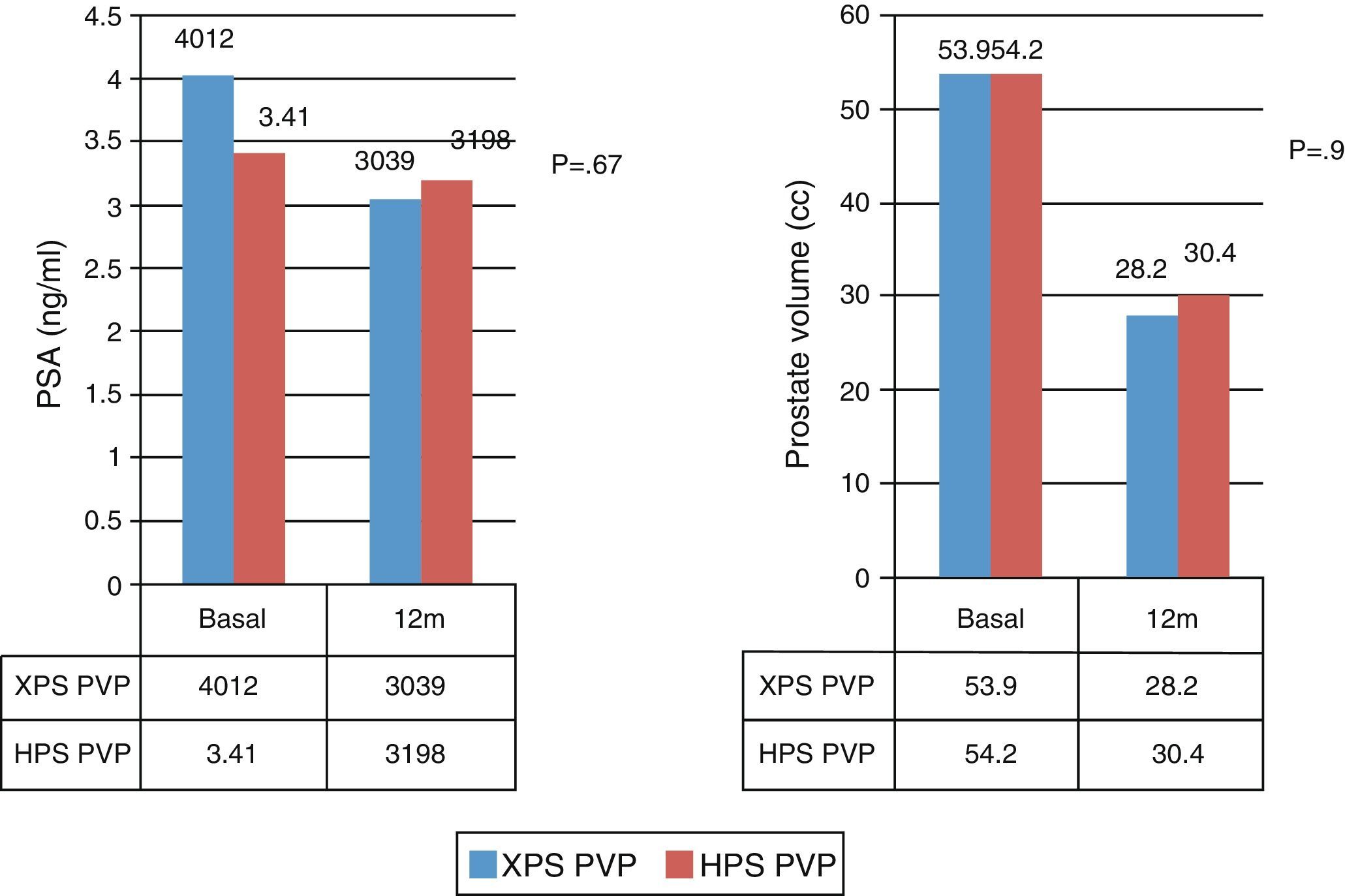
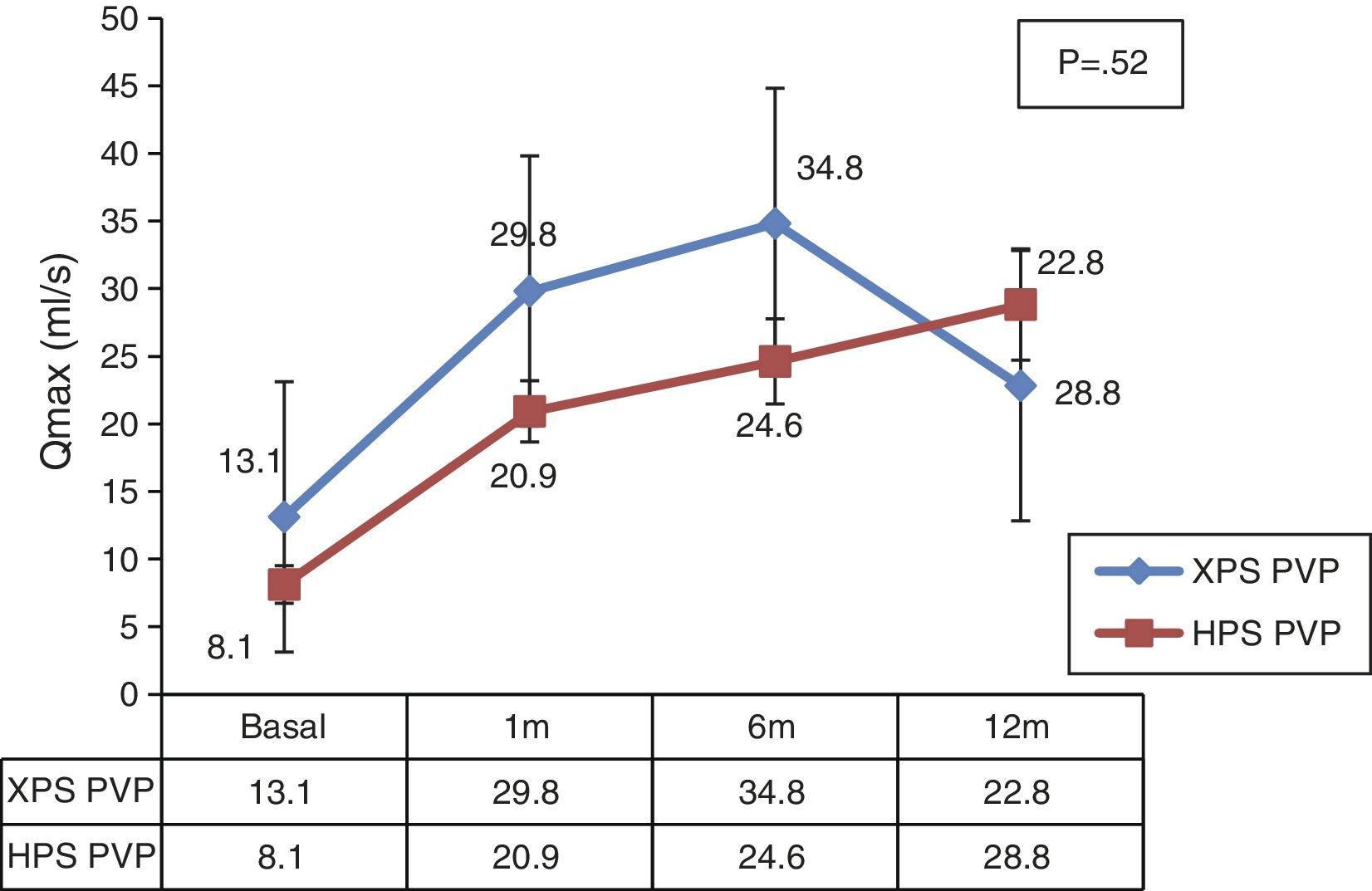
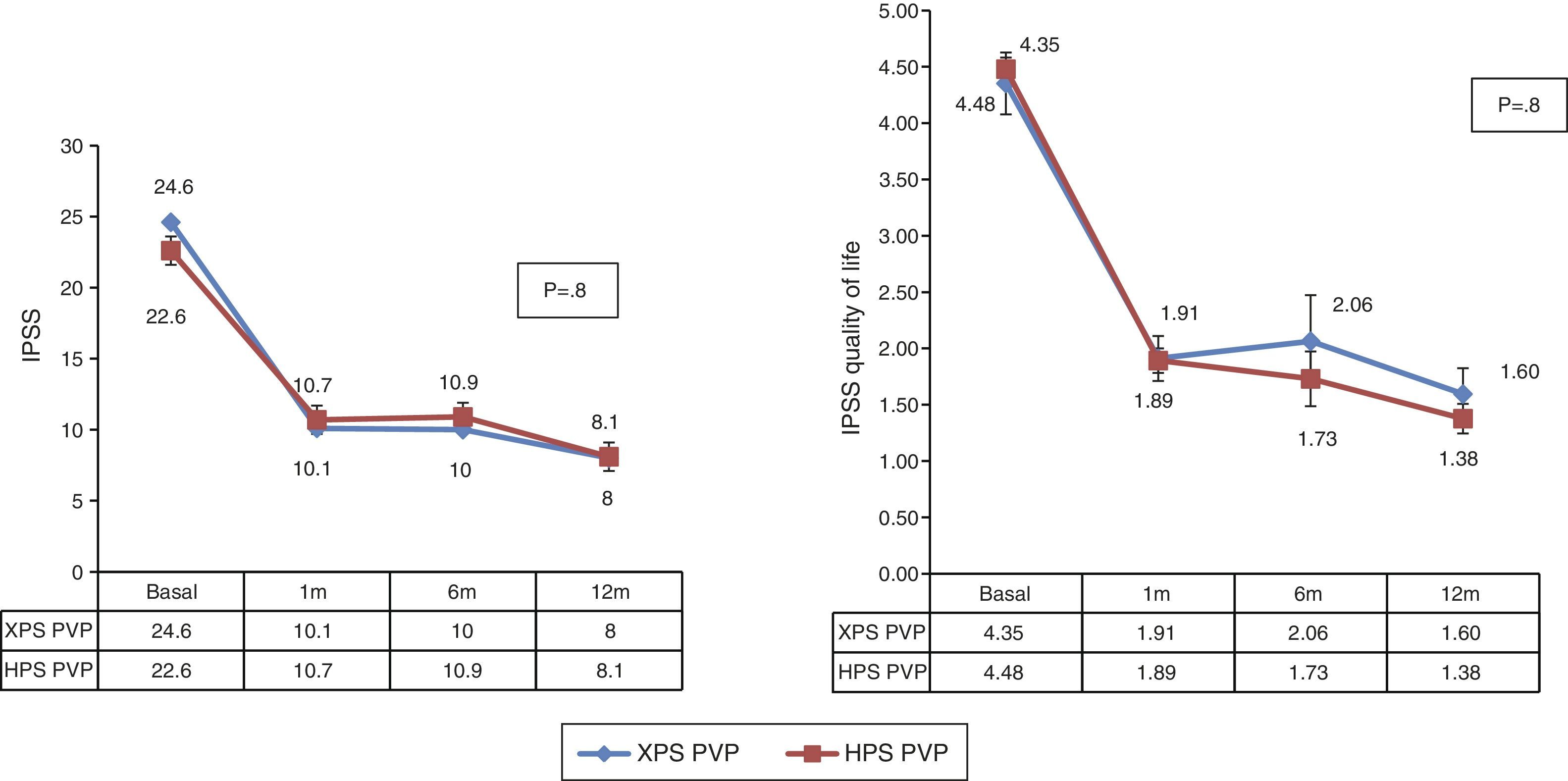
We observed significant differences both in the surgical time and effective laser time in favor of the XPS system. This advantage was 11% (48±15.7 vs. 53.8±16.2, p<.05) and 9% (32.8±11.7 vs. 36±11.6, p<.05), respectively. There were no statistically significant differences in the rest of the analyzed parameters.
ConclusionThe technical improvements in the XPS 180W system help reduce surgical time, maintaining the safety and efficacy profile offered by the HPS 120W system, with completely superimposable results at 1 year of follow-up.
La fotovaporización prostática con láser Greenlight, para el tratamiento quirúrgico de la hiperplasia benigna de próstata ha evolucionado rápidamente hasta el nuevo XPS 180W.
Demostramos anteriormente la eficacia y seguridad con el HPS 120W. El objetivo de este estudio ha sido evaluar los resultados funcionales y de seguridad, con un año de seguimiento, de la fotovaporización mediante el XPS 180W comparándolo con su predecesor.
Material y métodosEstudio de cohortes sobre una serie de 191 pacientes consecutivos sometidos a fotovaporización entre 01/2008 y 05/2013. Los criterios de inclusión fueron un IPSS >15 tras fracaso médico, un volumen prostático <80cc y un flujo máximo <15ml/s. Se evaluaron variables preoperatorias, intraoperatorias (energía empleada, tiempo de láser y tiempo total de la cirugía), complicaciones, horas de sonda, estancia y resultados funcionales (flujo máximo, IPSS, PSA y volumen prostático) a 3, 6 y 12 meses.
Se analiza la homogeneidad en las características preoperatorias de los dos grupos mediante técnicas de análisis univariante. Los resultados funcionales postoperatorios se evalúan mediante análisis de la varianza de medidas repetidas con modelos mixtos.
ResultadosSe realizaron 109 (57,1%) procedimientos mediante HPS 120W y 82 (42,9%) mediante XPS.
No se encontraron diferencias entre las características preoperatorias.
Se observaron diferencias significativas tanto en el tiempo quirúrgico como en el tiempo efectivo de láser a favor del sistema XPS, siendo esta ventaja de un 11% (48±15,7 vs. 53,8±16,2, p<0,05), y de un 9% (32,8±11,7 vs. 36±11,6, p<0,05), respectivamente. En el resto de los parámetros analizados no se encontraron diferencias estadísticamente significativas.
ConclusiónLas mejorías técnicas del sistema XPS 180W permiten reducir el tiempo quirúrgico manteniendo el perfil de seguridad y eficacia que ofrecía el sistema HPS 120W con unos resultados totalmente superponibles con un año de seguimiento.