During the beginning of the COVID-19 pandemic in our center, neither prehabilitation nor multimodal rehabilitation could be applied due to the excessive patient load on the health system and to reduce SARS-CoV-2 transmission. The objective of our study was to analyze the evolution, complications, and survival up to one year of patients who underwent radical cystectomy in our hospital from March 1st to May 31st, 2020 (period of the first wave COVID-19 pandemic in Spain). We also compared the results with cystectomized patients outside the pandemic period and with application of the ERAS (Enhanced Recovery After Surgery) protocol.
Material and methodsSingle-center, retrospective cohort study of patients scheduled for radical cystectomy from March 1st, 2020 to May 31st, 2020. They were matched with previously operated patients using a 1:2 propensity matching score. The matching variables were demographic data, preoperative and intraoperative clinical conditions.
ResultsA total of 23 radical cystectomies with urinary diversion were performed in the period described. In none of the cases the prehabilitation or the follow-up of our ERAS protocol could be applied, and this was the only difference we found between the 2 groups. Although the minimally invasive approach was more frequent in the pandemic group, the difference was not statistically significant. Three patients were diagnosed with COVID-19 during their admission, presenting severe respiratory complications and high in-hospital mortality. Apart from respiratory complications secondary to SARS-CoV-2, we also found statistically significant differences in other postoperative complications. The hospital stay increased by 3 days in the pandemic group.
ConclusionsPatients who underwent radical cystectomy at our center during the first wave of the COVID-19 pandemic had a higher number and severity of respiratory and non-respiratory complications. Discontinuation of the ERAS protocol was the main difference in treatment between groups.
Al inicio de la pandemia COVID-19 no se pudo implementar ni prehabilitación ni rehabilitación multimodal por sobrecarga del sistema sanitario. Nuestro objetivo fue analizar evolución, complicaciones y supervivencia hasta el año de pacientes sometidos a cistectomía radical en nuestro centro desde 1 de Marzo hasta 31 de Mayo de 2020 (primera ola). Comparamos resultados con pacientes también cistectomizados fuera de pandemia donde sí estaba instaurado el protocolo ERAS.
Material y MétodosEstudio de cohortes retrospectivo, unicéntrico, de pacientes programados para cistectomía radical desde 1 de marzo de 2020 hasta 31 de mayo de 2020; se emparejaron con pacientes intervenidos anteriormente a través de score de emparejamiento por propensión 1:2. Las variables de emparejamiento fueron datos demográficos, condiciones clínicas preoperatorias e intraoperatorias.
ResultadosSe realizaron 23 cistectomía en este periodo; en ningún caso se aplicó ni prehabilitación ni seguimiento del protocolo ERAS, y ésta fue la única diferencia en el tratamiento entre grupos. Tres pacientes se diagnosticaron de COVID-19 durante su ingreso presentando complicaciones respiratorias graves y alta mortalidad intrahospitalaria. La tasa de transfusión sanguínea fue mayor en el grupo pandemia. La estancia hospitalaria aumentó en 3 días en grupo pandemia.
ConclusionesLos pacientes sometidos a cistectomía en nuestro centro durante la primera ola de pandemia COVID-19 presentaron complicaciones respiratorias y no respiratorias en mayor número y más graves que los cistectomizados fuera de este periodo. La no aplicación del protocolo ERAS fue la principal diferencia en el tratamiento entre grupos.
COVID-19 disease caused by the SARS-CoV-2 virus provoked an unprecedented global health emergency. On March 11, 2020, the World Health Organization (WHO) declared a pandemic situation following the exponential increase in the number of cases, and restrictive policies were initiated to reduce the risk of SARS-CoV-2transmission.1–3 Spain has been among the European countries most affected by the COVID-19 pandemic, especially during March, April, and May 2020 (first wave).4,5
The first wave of the COVID-19 pandemic resulted in the postponement of 29%–53% of genitourinary oncologic surgery.6 Nevertheless, urgent, and non-deferrable oncological surgeries had to be performed.7 The European Association of Urology (EAU) published recommendations to prioritize oncologic surgery and to adequately select operable patients, with the aim of optimizing healthcare resources and reducing the risk of hospital- acquired transmission of COVID-19.8,9
Radical cystectomy (RC) is the treatment of choice in certain bladder tumors. The high comorbidity among these patients, added to the complexity of this surgery, results in a complication rate of 65%–70%.10–12 Furthermore, the delay of more than 90 days from tumor diagnosis in radical surgery has been shown to worsen the prognosis of the disease and increase mortality.13,14 Surgical delay is only contemplated in cases where neoadjuvant therapy is indicated, in which it is advised that surgery should not be delayed for more than two months after completion of chemotherapy.15
The primary objective of our study was to analyze the outcomes, complications and survival of patients undergoing RC in our hospital during the period of the first wave of the COVID-19 pandemic (March 1, 2020, to May 31, 2020) at three time points: at admission, after 30 days and one year after hospital discharge. As a secondary objective, we compared the evolution of these patients with a historical cohort of patients who also underwent RC, but their interventions took place outside this period and included an Enhanced Recovery After Surgery (ERAS) program.
Material and methodsThe ERAS program of our hospital includes a database where we have been collecting variables since 2016. Based on this registry, a single-center, retrospective cohort study was performed, including data collected during admission, at 30 days, and one year after hospital discharge, of patients who underwent RC with urinary diversion during the months of March, April, and May 2020 (first wave of the pandemic).
This study was conducted following the Helsinki declaration, with approval from the ethics committee of our hospital.
The study variables were demographic characteristics, physical status according to the American Society of Anesthesiologist (ASA) scale, comorbidities, laboratory parameters, tumor staging and neoadjuvant therapy administration. Perioperative variables such as surgical approach, type of urinary diversion and anesthesia, estimated blood loss and need for transfusion of blood products, and surgical time were also recorded. Postoperative data included complications registered according to the Clavien-Dindo classification, reinterventions, mean length of stay, number of readmissions within 30 days, and survival within one year after cystectomy.
All our patients were evaluated by the multidisciplinary oncology committee and the recommendations of the EAU were followed for prioritization of surgeries and patient selection.7
As of April 13, 2020, screening with PCR (Polymerase Chain Reaction) for SARS-CoV-2 was systematically implemented in our center within 48−72 h prior to hospital admission. During admission and after discharge, nasopharyngeal swab test was repeated only in patients with close COVID-19 contacts or with symptomatology compatible with COVID-19.
To compare our group of patients with a historical cohort (control group), we extracted from the database information on 46 patients who underwent RC prior to the first wave of the pandemic and who could be matched by the aforementioned preoperative and intraoperative variables.
Statistical analysisCategorical variables were presented as absolute and relative frequency (percentage). Continuous variables were presented as means (standard deviation) or medians (interquartile range). For comparison of means, the t-test or Mann–Whitney U test were used, depending on the normality of the distribution. The Chi-squared test or Fisher's exact test were used to compare proportions.
A matching method was used to compare between patients operated on during the first wave of the COVID-19 pandemic and patients operated on prior to it. A 1:2 matching (one case for two controls) was performed by calculating a propensity score matching. A goodness-of-fit test was performed for the matching of the two cohorts.
Survival analysis was performed until one year of follow-up and the Log-Rank test was performed to compare mortality between the two groups.
The statistical software used was IBM SPSS Statistics 20.0. P was considered statistically significant below 0.05.
ResultsIn the 3 months of the study, 23 patients underwent RC with urinary diversion. The first 11 patients did not undergo preoperative screening for SARS-CoV-2; the following 12 patients (as of April 13, 2020) did undergo PCR for SARS-CoV-2 48−72 h prior to admission.
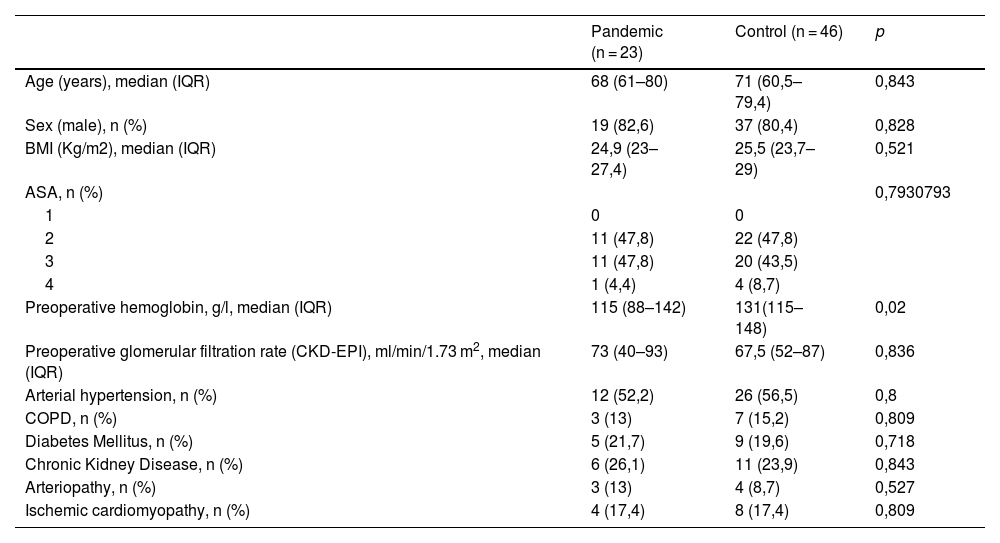
This cohort of 23 patients (pandemic group) was compared with another cohort of 46 patients (control group). No statistically significant differences were found between the two groups in terms of demographic and clinical variables (Table 1).
Demographic and medical history.
| Pandemic (n = 23) | Control (n = 46) | p | |
|---|---|---|---|
| Age (years), median (IQR) | 68 (61–80) | 71 (60,5–79,4) | 0,843 |
| Sex (male), n (%) | 19 (82,6) | 37 (80,4) | 0,828 |
| BMI (Kg/m2), median (IQR) | 24,9 (23–27,4) | 25,5 (23,7–29) | 0,521 |
| ASA, n (%) | 0,7930793 | ||
| 1 | 0 | 0 | |
| 2 | 11 (47,8) | 22 (47,8) | |
| 3 | 11 (47,8) | 20 (43,5) | |
| 4 | 1 (4,4) | 4 (8,7) | |
| Preoperative hemoglobin, g/l, median (IQR) | 115 (88–142) | 131(115–148) | 0,02 |
| Preoperative glomerular filtration rate (CKD-EPI), ml/min/1.73 m2, median (IQR) | 73 (40–93) | 67,5 (52–87) | 0,836 |
| Arterial hypertension, n (%) | 12 (52,2) | 26 (56,5) | 0,8 |
| COPD, n (%) | 3 (13) | 7 (15,2) | 0,809 |
| Diabetes Mellitus, n (%) | 5 (21,7) | 9 (19,6) | 0,718 |
| Chronic Kidney Disease, n (%) | 6 (26,1) | 11 (23,9) | 0,843 |
| Arteriopathy, n (%) | 3 (13) | 4 (8,7) | 0,527 |
| Ischemic cardiomyopathy, n (%) | 4 (17,4) | 8 (17,4) | 0,809 |
IQR: interquartile range; BMI: body mass index; ASA: physical status classification according to the American Society of Anesthesiologists; CKD-EPI: equation to estimate glomerular filtration rate from the Chronic Kidney Disease Epidemiology Collaboration; COPD: chronic obstructive pulmonary disease.
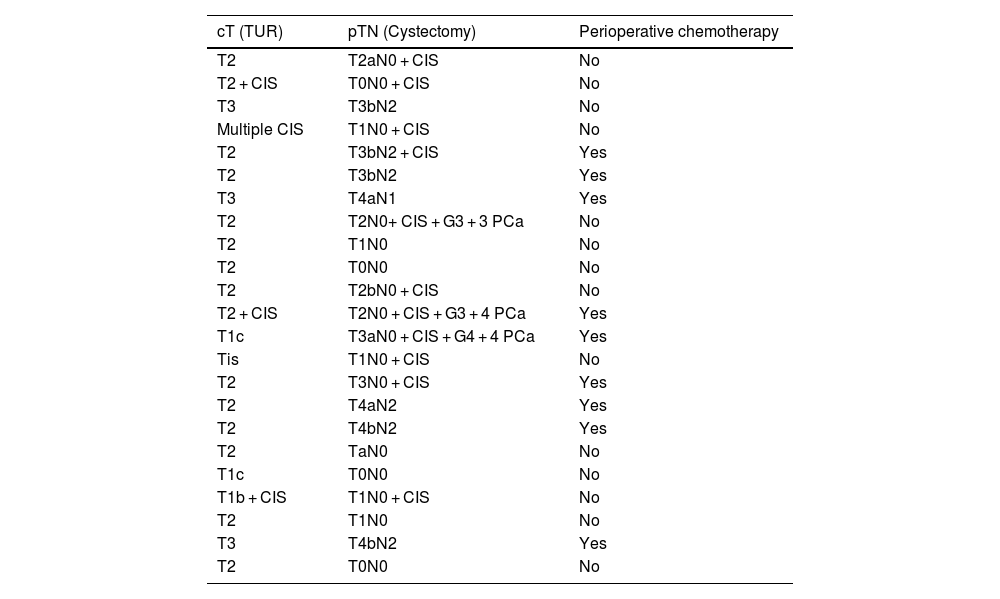
Table 2 summarizes tumor staging and data on the presence or absence of perioperative chemotherapy.
Tumor staging.
| cT (TUR) | pTN (Cystectomy) | Perioperative chemotherapy |
|---|---|---|
| T2 | T2aN0 + CIS | No |
| T2 + CIS | T0N0 + CIS | No |
| T3 | T3bN2 | No |
| Multiple CIS | T1N0 + CIS | No |
| T2 | T3bN2 + CIS | Yes |
| T2 | T3bN2 | Yes |
| T3 | T4aN1 | Yes |
| T2 | T2N0+ CIS + G3 + 3 PCa | No |
| T2 | T1N0 | No |
| T2 | T0N0 | No |
| T2 | T2bN0 + CIS | No |
| T2 + CIS | T2N0 + CIS + G3 + 4 PCa | Yes |
| T1c | T3aN0 + CIS + G4 + 4 PCa | Yes |
| Tis | T1N0 + CIS | No |
| T2 | T3N0 + CIS | Yes |
| T2 | T4aN2 | Yes |
| T2 | T4bN2 | Yes |
| T2 | TaN0 | No |
| T1c | T0N0 | No |
| T1b + CIS | T1N0 + CIS | No |
| T2 | T1N0 | No |
| T3 | T4bN2 | Yes |
| T2 | T0N0 | No |
TUR: transurethral resection; CIS: carcinoma in situ; PCa: prostate cancer.
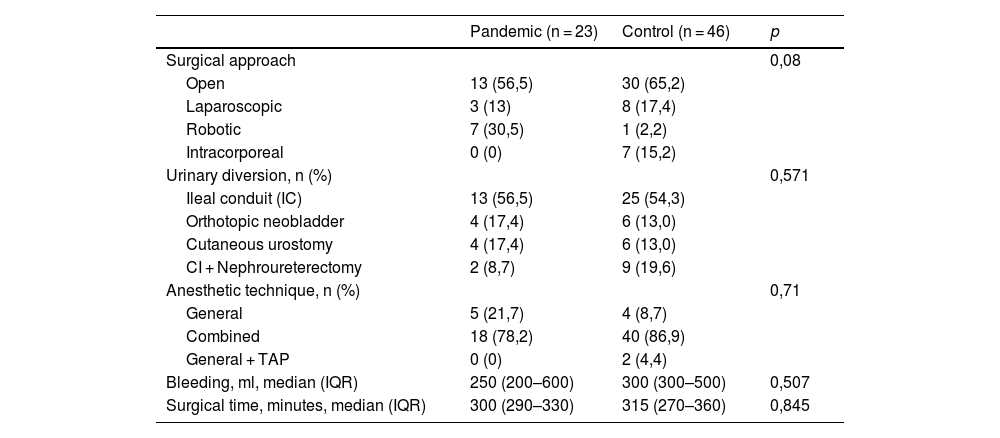
Intraoperative variables were comparable between the two groups. There was a greater tendency to a minimally invasive approach (laparoscopic or robotic) in the pandemic group than in the control group (43.5% vs. 34.8%, p 0.08) without being statistically significant (Table 3). Surgeons and surgical times were the same in both groups.
Intraoperative data.
| Pandemic (n = 23) | Control (n = 46) | p | |
|---|---|---|---|
| Surgical approach | 0,08 | ||
| Open | 13 (56,5) | 30 (65,2) | |
| Laparoscopic | 3 (13) | 8 (17,4) | |
| Robotic | 7 (30,5) | 1 (2,2) | |
| Intracorporeal | 0 (0) | 7 (15,2) | |
| Urinary diversion, n (%) | 0,571 | ||
| Ileal conduit (IC) | 13 (56,5) | 25 (54,3) | |
| Orthotopic neobladder | 4 (17,4) | 6 (13,0) | |
| Cutaneous urostomy | 4 (17,4) | 6 (13,0) | |
| CI + Nephroureterectomy | 2 (8,7) | 9 (19,6) | |
| Anesthetic technique, n (%) | 0,71 | ||
| General | 5 (21,7) | 4 (8,7) | |
| Combined | 18 (78,2) | 40 (86,9) | |
| General + TAP | 0 (0) | 2 (4,4) | |
| Bleeding, ml, median (IQR) | 250 (200–600) | 300 (300–500) | 0,507 |
| Surgical time, minutes, median (IQR) | 300 (290–330) | 315 (270–360) | 0,845 |
IQR: interquartile range; TAP: transversus abdominis plane block.
Three of the 23 operated patients became infected with COVID-19 during hospital stay; this was confirmed by a positive PCR test for SARS-CoV-2.
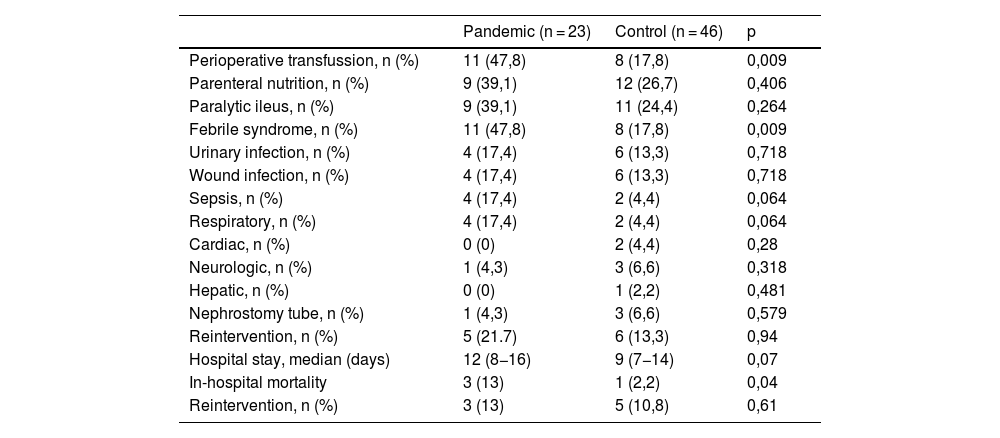
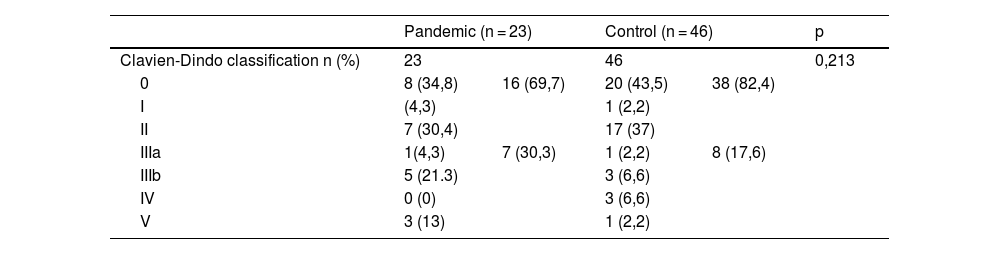
Tables 4 and 5 show postoperative complications and their severity, with febrile syndrome being the most frequent. Almost half (47.8%) of the patients in the pandemic group presented fever (>37.5 °C) at some time during the postoperative period, the most prevalent cause being urinary and respiratory infections. We found a higher percentage of respiratory complications in the pandemic group than in the control group, although the difference was not statistically significant (17.4% vs. 4.4%, p = 0.06). Five patients (21.7%) underwent surgical reintervention during admission: three for evisceration, one for suture dehiscence and another one for bowel obstruction, all of them with good postoperative outcome.
Postoperative complications.
| Pandemic (n = 23) | Control (n = 46) | p | |
|---|---|---|---|
| Perioperative transfussion, n (%) | 11 (47,8) | 8 (17,8) | 0,009 |
| Parenteral nutrition, n (%) | 9 (39,1) | 12 (26,7) | 0,406 |
| Paralytic ileus, n (%) | 9 (39,1) | 11 (24,4) | 0,264 |
| Febrile syndrome, n (%) | 11 (47,8) | 8 (17,8) | 0,009 |
| Urinary infection, n (%) | 4 (17,4) | 6 (13,3) | 0,718 |
| Wound infection, n (%) | 4 (17,4) | 6 (13,3) | 0,718 |
| Sepsis, n (%) | 4 (17,4) | 2 (4,4) | 0,064 |
| Respiratory, n (%) | 4 (17,4) | 2 (4,4) | 0,064 |
| Cardiac, n (%) | 0 (0) | 2 (4,4) | 0,28 |
| Neurologic, n (%) | 1 (4,3) | 3 (6,6) | 0,318 |
| Hepatic, n (%) | 0 (0) | 1 (2,2) | 0,481 |
| Nephrostomy tube, n (%) | 1 (4,3) | 3 (6,6) | 0,579 |
| Reintervention, n (%) | 5 (21.7) | 6 (13,3) | 0,94 |
| Hospital stay, median (days) | 12 (8−16) | 9 (7−14) | 0,07 |
| In-hospital mortality | 3 (13) | 1 (2,2) | 0,04 |
| Reintervention, n (%) | 3 (13) | 5 (10,8) | 0,61 |
The rate of patients with preoperative anemia was higher in the pandemic group (37% vs. 20%, p < 0.05), and although no differences were evidenced in intraoperative bleeding (250 ml vs. 300 ml, p = 0.57), the transfusion rate was also higher in the pandemic group (47.8% vs. 17.8%, p < 0.05).
In-hospital mortality was higher in the 23 patients in the pandemic group than in the control group, (13% vs 2.2%, p = 0.04). Two of the 3 deaths in the pandemic group were secondary to respiratory complications in 2 patients who were infected by COVID. The mean hospital stay was 12 days (CI 8−16), 3 days longer than in the control group (p = 0.07).
There were no statistically significant differences between the two groups in 30-day post-discharge complications. Urinary tract infection (UTI) was the most frequent complication (35%). There were no respiratory complications. The readmission rate was 14.3%, in all cases due to UTI, with no differences with respect to the control group.
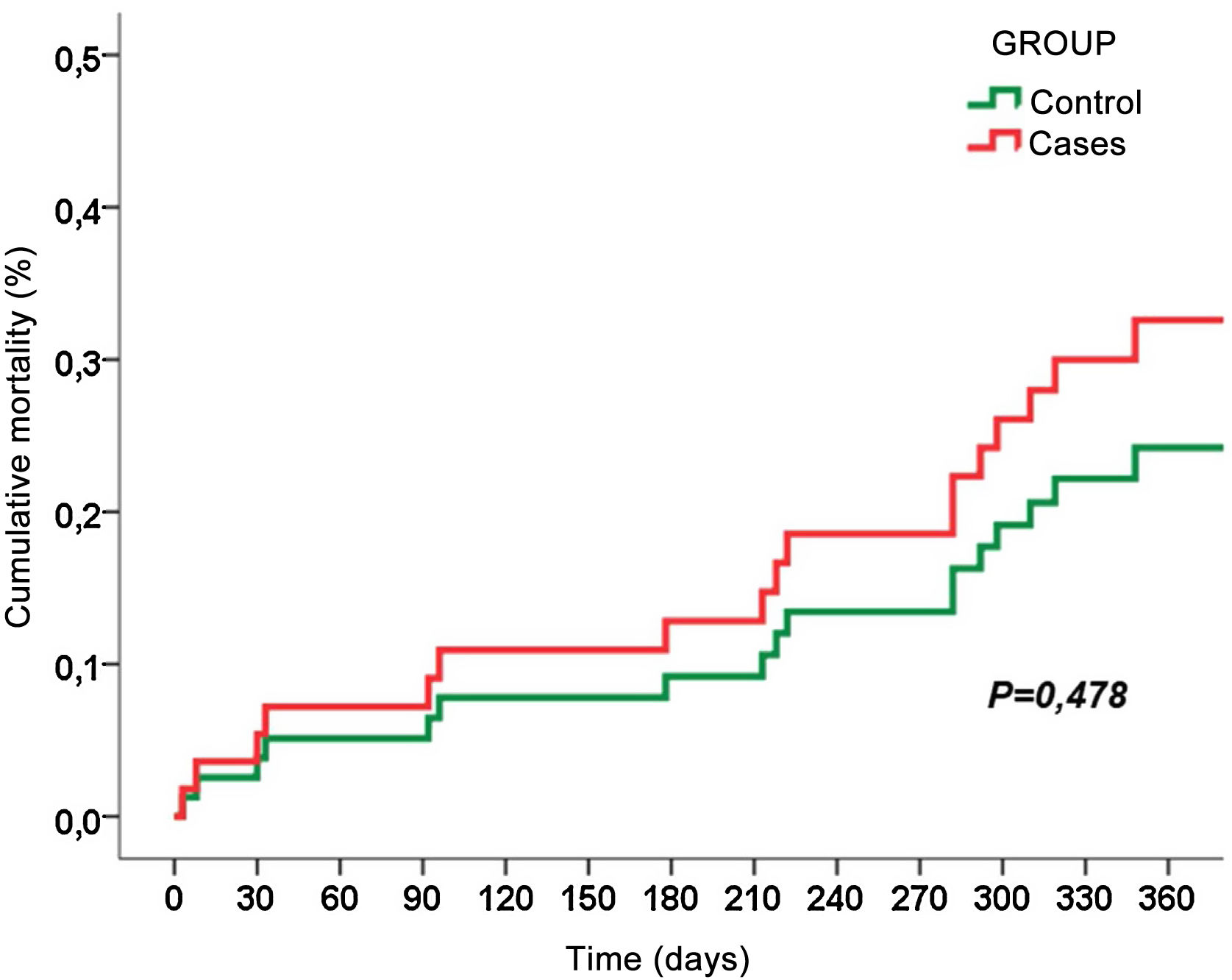
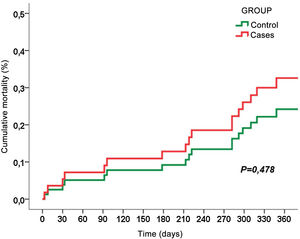
Fig. 1 shows the cumulative mortality at one year after surgery, and we found no statistically significant differences between the two groups (30.4% vs. 21.7%, p = 0.42).
DiscussionThe main objective of our study was to analyze the outcomes of patients undergoing RC during the first period of the COVID-19 pandemic. We found statistically significant differences in postoperative complications between the two groups, being greater in number and severity in the pandemic group.
Other published series have also reported that patients who underwent surgery during the first wave of the pandemic had a higher risk of complications and mortality.16–19 The severity and high number of patients affected by COVID-19 during the months of March, April and May 2020 caused maximum pressure on the national health care system. Despite this, the number of cystectomies performed in our hospital during the first wave was similar to the number of RCs performed during the same period of the previous year. The creation of a COVID-19 multidisciplinary committee and the rapid and strict adaptation to the recommendations of the EAU were essential to minimize the risk of contagion.
The shortage of diagnostic tests at the beginning of the pandemic did not allow screening of the population with clinical suspicion of infection, nor preoperative PCR screening of patients who underwent surgery during the beginning of the pandemic.20,21 Since PCR testing for SARS-CoV-2 was performed prior to hospital admission, in our hospital this occurred from April 13, 2020, only 1 case out of 13 patients undergoing cystectomy was positive in the preoperative screening, representing a preoperative infection rate of 7.7%.
Enhanced recovery after surgery (ERAS) protocols have been shown to improve the outcomes of patients undergoing RC and to reduce complications and hospital stay.22 We implemented the ERAS protocol in RC in our hospital since 2016. Unfortunately, in March 2020, with the cancellation of nonessential surgical procedures, the ERAS protocol was also suspended. Therefore, during the first wave our patients did not benefit from either prehabilitation or multimodal rehabilitation. Preoperative visits were carried out through telemedicine avoiding the patient's visits to the hospital. The Patient Blood Management (PBM) approach was also suspended, so neither anemia nor preoperative iron deficiency were treated. Preoperative hemoglobin was lower in the pandemic group than in the control group (115 vs. 131 g/l, p 0.02). The pandemic group received more transfusions than the control group (47.8% vs. 17.8%, p 0.01). Along these lines, a higher percentage of patients in the first wave required total parenteral nutrition (TPN) than the control group, although without significant differences (39.1% vs. 26.7% p 0.41). We also associated this fact with the suspension of prehabilitation and multimodal rehabilitation protocols.
There were no differences between the two groups with respect to perioperative chemotherapy. We did not observe complications derived from chemotherapy treatment, and none of the patients with severe complications had undergone neoadjuvant treatment.
During the first wave of the pandemic, minimally invasive surgery was prioritized aiming to shorten hospital stay. However, there were doubts concerning whether the aerosols produced by laparoscopic/robotic surgery could increase the risk of virus transmission. In our hospital we strongly believe in minimally invasive surgery combined with enhanced recovery protocols. Our preferred approach is robotic with intracorporeal reconstruction. We had only one robotic platform until one year ago, and it was also necessary for other surgeries (prostatectomy and complex partial nephrectomy). This, added to the high volume of cystectomies/year/center, justifies the number of open approaches. We must mention that none of the surgeries were performed with an intracorporeal approach, probably due to the prolonged surgical time. Despite a greater use of robotic approach during the pandemic, we did not find significant differences in surgical time between the two groups: surgeries were not educational and were performed by expert surgeons.
Oncologic patients have a compromised immune function and, therefore, a higher risk of infection compared to the general population.23,24 Of the 3 patients (13%) who became infected with COVID-19 during their hospital stay, 2 of them were admitted without preoperative PCR (they were either asymptomatic carriers of SARS-CoV-2 or became infected in the hospital), and the third had a negative preoperative PCR, with healthcare workers being the possible vectors of hospital contagion.
According to Lei et al. elective surgery in asymptomatic or incubating SARS-COV-2 patients could accelerate and exacerbate the progression of COVID-19 disease, reaching a mortality of 20.5%.25 In-hospital mortality tended to be higher in the pandemic group than in the control group, although the difference was not statistically significant (13% vs. 2.2%, p 0.05). None of the 3 deceased patients had undergone preoperative PCR tests, none had previous respiratory pathology, none were smokers and all of them died of respiratory causes. One patient died 72 h after cystectomy due to respiratory failure and massive aspiration. As he had not undergone PCR prior to admission, we cannot rule out that he was an asymptomatic COVID-19 carrier on admission. It is possible that the surgery, the consequent immunosuppression, and mechanical ventilation rapidly precipitated the poor outcome. The other two patients died of bilateral SARS-CoV-2 pneumonia and multiorgan failure. Therefore, we believe that having preoperative screening by PCR was critical to improve the safety of the surgical patient and to control in-hospital transmissibility of SARS-CoV-2 virus.
In the first 30 days after hospital discharge, all complications were due to UTI and no patient was infected with COVID-19; it should be considered that this was a period of mandatory home confinement.
Fig. 1 shows the cumulative mortality from surgery and within one year of postoperative follow-up, with no significant differences between the two groups (30.4% vs. 21.7%, p 0.42). Of the 7 patients who died during the first year, 3 died during hospital admission, another 3 died due to progression of cancer and 1 patient died of septic shock due to bilateral SARS-CoV-2 pneumonia 9 months after surgery.
The limitations of our study include the retrospective design, which may lead to missing data. In addition, the study sample is small and represents patients very well selected by the COVID-19 multidisciplinary committee. Conflicts of interest: none.
In conclusion, patients who underwent radical cystectomy with urinary diversion in our hospital during the period of the first wave of the COVID-19 pandemic presented a higher number and more severe postoperative complications than patients who underwent radical cystectomy outside this period. The coexistence of the COVID-19 pandemic and the discontinuation of the ERAS protocol were the only two differences between groups. The respiratory complications and increased mortality are attributable to the virus infection, but the remaining complications can be justified by the suspension of the enhanced recovery protocol.