The treatment of choice for high-risk non-muscle invasive bladder cancer (NMIBC) is bacillus Calmette-Guérin (BCG). However, when this fails, the indicated treatment is radical cystectomy. In recent years, trials are being developed with various drugs to avoid this surgery in patients with BCG failure. The aim of this article is to update the treatments under study for bladder preservation in this patient population.
Material and MethodsNon-systematic review, searching PubMed with the terms "Bladder cancer", "Non-muscle invasive bladder cancer", "NMIBC", "BCG", "BCG-refractory", "Mitomycin C", "MMC", "Hyperthermia", "Electromotive Drug Administration", "EMDA" We used the search engines clinicaltrials.gov and clinicaltrialsregister.eu to find clinical trials.
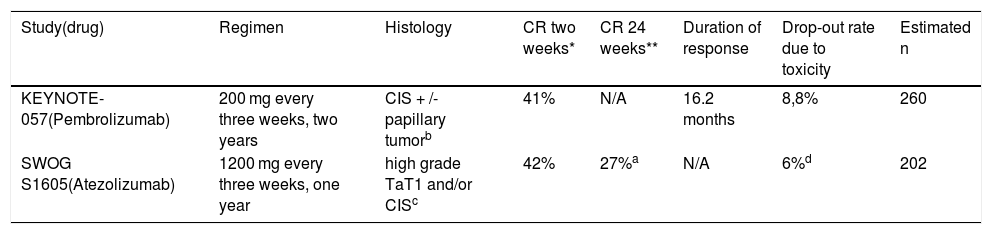
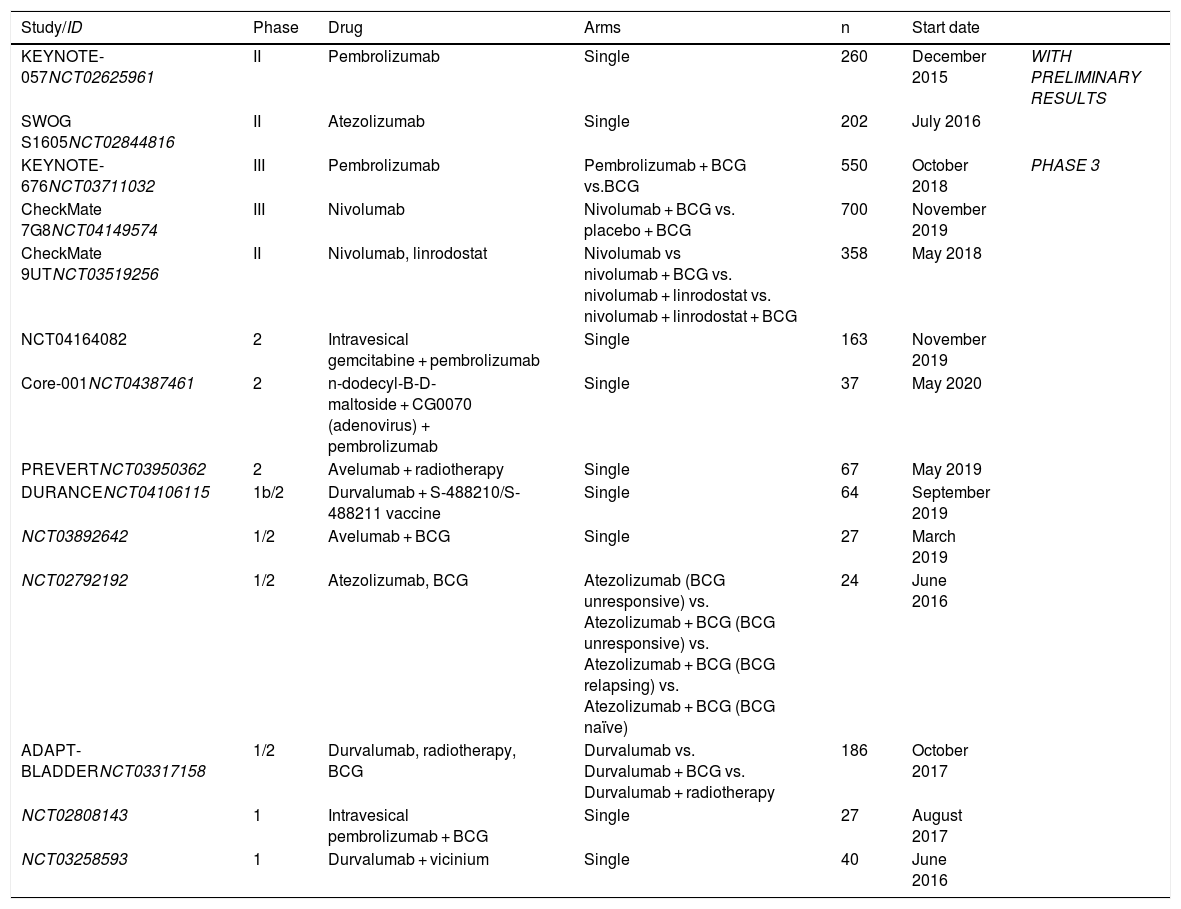
ResultsThe only intravesical drug approved by the Food and Drug Administration (FDA) for carcinoma in situ (CIS) after failure to BCG is valrubicin. Recently, the FDA has approved intravenous pembrolizumab, following the publication of preliminary data from the KEYNOTE-057 study. Atezolizumab has demonstrated similar preliminary efficacy results. Only microwave-induced chemohyperthermia and EMDA-MMC (electromotive drug administration) are recognized as alternatives in European guidelines. Other options under investigation are taxanes and gemcitabine, alone or in combination, recombinant viruses and device-assisted intravesical chemohyperthermia.
ConclusionsThe results of new drugs are promising, with a large number of trials underway. Knowing the mechanisms of resistance to BCG is essential to explore new therapeutic options.
El tratamiento de elección para el cáncer vesical no músculo infiltrante (CVNMI) de alto riesgo es el bacilo de Calmette-Guérin (BCG). Sin embargo, cuando éste falla, el tratamiento indicado es la cistectomía radical. En los últimos años se están desarrollando ensayos con diversos fármacos para evitar esta cirugía en pacientes con fracaso a BCG. El objetivo de este artículo es llevar a cabo una puesta al día de los tratamientos en estudio para la preservación vesical en esta población de pacientes.
Material y MétodosRevisión no sistemática, realizando una búsqueda en PubMed con los términos “Bladder cancer”, “Non-muscle invasive bladder cancer”, “NMIBC”, “BCG”, “BCG-refractory”, “Mitomycin C”, “MMC”, “Hyperthermia”, “Electromotive Drug Administration”, “EMDA” Empleamos los buscadores clinicaltrials.gov y clinicaltrialsregister.eu para localizar ensayos clínicos.
ResultadosEl único fármaco intravesical aprobado por la Food and Drug Administration (FDA) para carcinoma in situ (CIS) tras fracaso a BCG es la valrubicina. Recientemente la FDA ha aprobado pembrolizumab intravenoso, tras la publicación de los datos preliminares del estudio KEYNOTE-057. Atezolizumab ha demostrado unos resultados preliminares similares de eficacia. En las guías europeas se reconoce como alternativa únicamente la quimiohipertermia inducida por microondas y EMDA-MMC (electromotive drug administration). Otras alternativas en investigación son los taxanos y la gemcitabina, solos o en combinación, los virus recombinantes y la quimiohipertermia intravesical asistida por dispositivos.
ConclusionesLos resultados de los nuevos fármacos son prometedores, con gran número de ensayos en marcha. Conocer los mecanismos de resistencia a BCG es imprescindible para la exploración de nuevas alternativas terapéuticas.