In this short review, our objective was to describe few new applications of a natural polysaccharide extracted from algae, the alginates. Firstly, the chemical composition is recalled and the main techniques used for characterization are cited. The role of guluronic acid blocks (GG blocks) is particularly important for gel mechanical properties in presence of calcium counterions. Then, the main applications in food and biomedicine are briefly mentioned followed by tentative applications in packaging, paper, textil and wound dressing which are described. Especially, fine fibers are produced now industrially under mixed sodium/calcium ionic form.
En esta corta revisión, nuestro objetivo fue describir algunas nuevas aplicaciones de un polisacárido natural extraído de algas: el alginato. Primeramente, la composición química es revisada, además de citar las principales técnicas utilizadas para su caracterización. El papel de los bloques de ácido gulurónico (bloques GG) es particularmente importante para las propiedades mecánicas del gel en presencia de Ca como contraión. Después, se mencionan brevemente las principales aplicaciones en el área de alimentos y biomedicina, seguida de aplicaciones experimentales en embalaje, papel, textiles, y vendajes de heridas, las cuales son descritas. Especialmente, fibras finas de alginato son ahora producidas industrialmente en una forma iónica mixta de sodio/calcio.
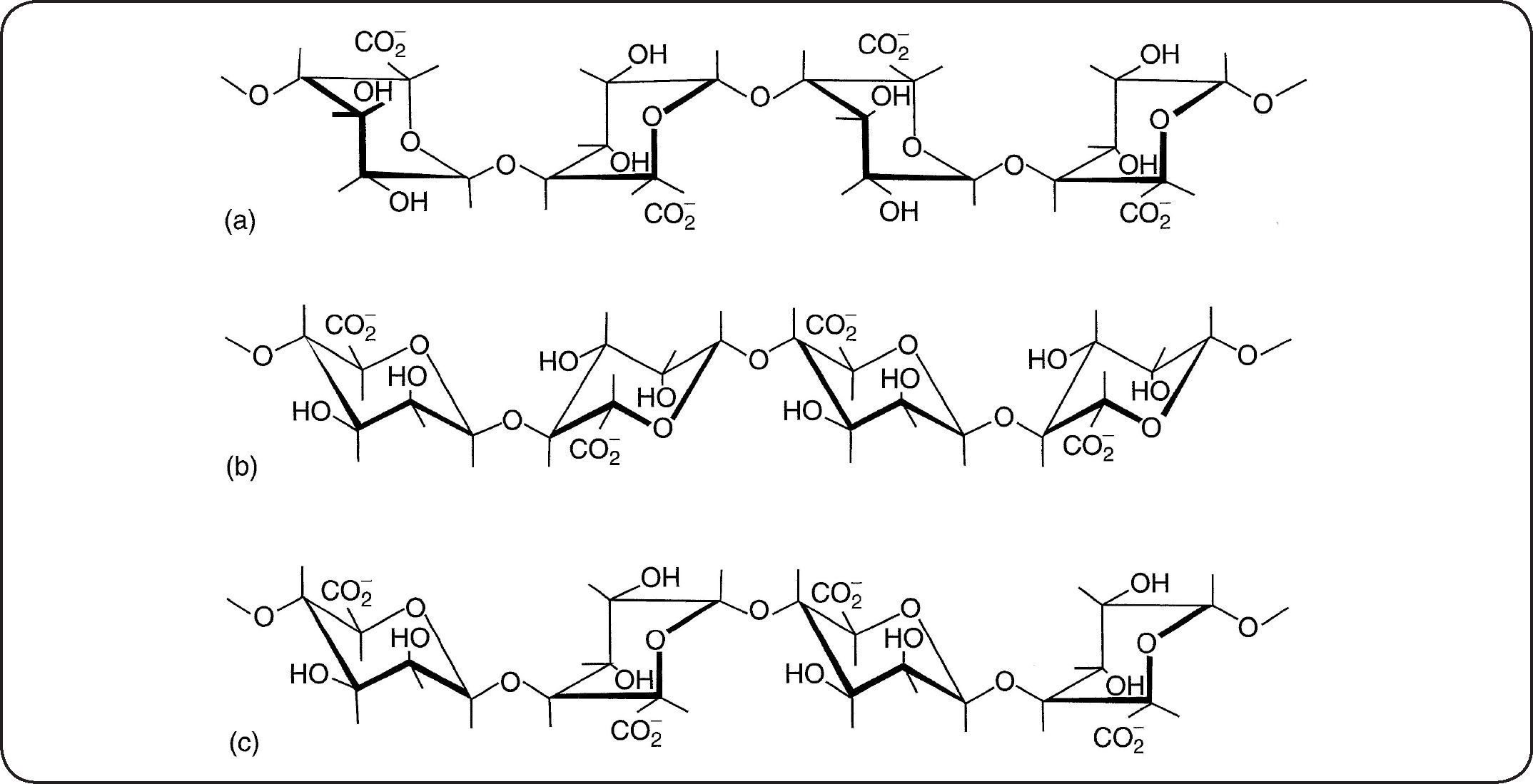
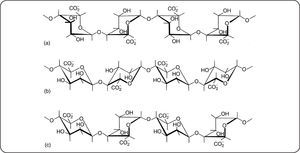
Marine algae constitute a large source of natural polysaccharides having original physical properties. Algae also contain a large variety of nutriments such as vitamins, salts, iodine, sterols…1 There is a large variety of such organisms which are exploited for a long time mainly for food applications. The brown algae (Phaeophyceae such as Fucus, Laminaria, Ascophyllum) contain large amounts of alginate in their cell walls1,2. The quantity (between 25 and 45% dried weight of crude algae) and quality of the alginates depend on the algae species, the type and age of the tissues and methods used for extraction. They are linear polymers composed of (1→4)-α-L-guluronic acid blocks (GG) (Figure 1a), (1→4)-β-D-mannuronic acid blocks (MM)(Figure 1b), and additionally, of heteropolymeric sequences of M and G (MG blocks) (Figure 1c).
Alginates are mainly used in the food and biomedical domains but in this paper we will focus on bioplastic applications, a less common field developed in the literature, i.e. applications of alginates in the domains of packaging, textiles, paper and wound dressing.
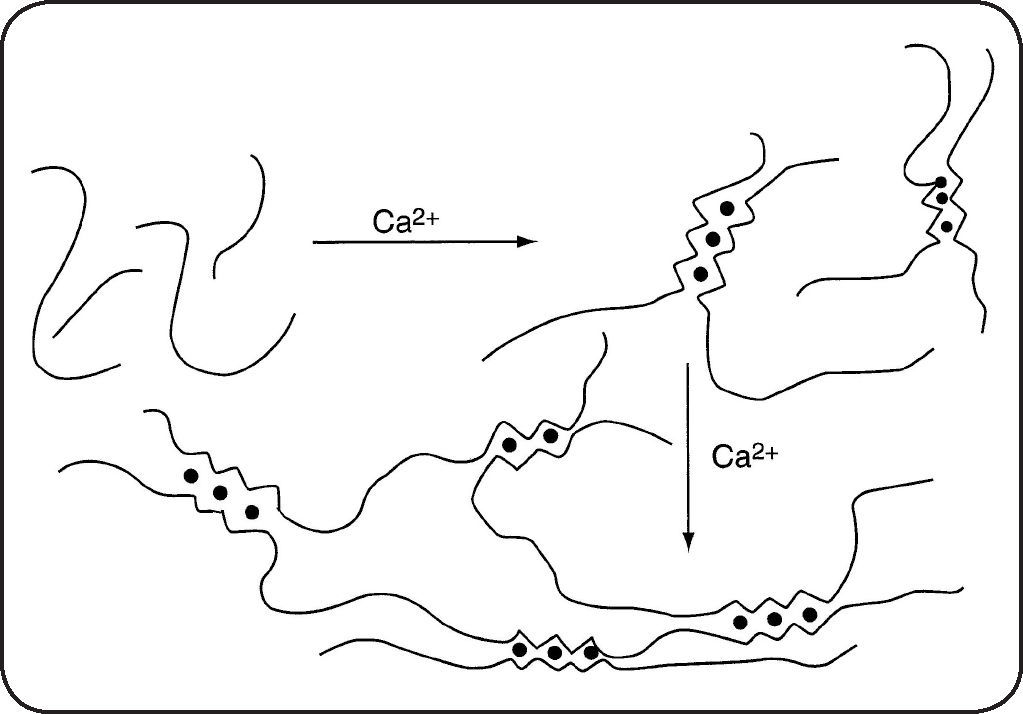
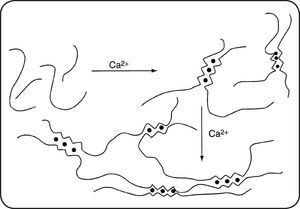
Main characteristicsThe biological and physical properties of alginates in aqueous media depend not only on the M/G ratio (obtained by proton NMR) but also on the distribution of M and G units along the chain3–6. Purified alginates have widespread industrial uses especially due to their ability to form hydrogels, beads, fibers or films mainly in presence of calcium attributed to the presence of zones rich in GG blocks following the model of an egg-box7. Nevertheless, it must be mentioned that MM and MG blocks also interact with calcium counterions. A schematic representation of gelation for progressive and cooperative fixation of calcium is given in Figure 2.
The stiffness of alginates gels is directly related to the GG block content8. Under their monovalent salt form (especially sodium form), alginates are perfectly water soluble and increase of solvent viscosity upon dissolution causing an interesting thickening character (in relation with the semirigid conformation of this copolymer) which found valuable applications. In addition, the ion-exchange properties of alginates are important due to specific strong interactions with counterions such as Ba, Sr and Ca giving a gel which is reversible in presence of large excess of monovalent salts or complexing agent (EDTA, sodium oxalate, sodium citrate). Alginic acid or alginate in acidic medium gives also specific gels based on H-bond network 9. Another important parameter is the distribution of the molar masses and the average molar mass (obtained by steric exclusion chromatography) which also control the viscosity of solution and stiffness of the gels. It is important to mention that specific low molar mass oligomers may be obtained by partial hydrolysis in acidic conditions followed by fractionation at controlled pH to isolate GG and MM blocks10,11.
Alginates applicationsAlginates in FoodsUp to now, the main applications of alginates were based mainly on their gel-forming ability and used as food additives in jams, jellies…. to improve and stabilize the structure of food. In this domain, alginates are also widely used as additives capable of viscosifying, stabilizing, emulsifying solutions12.
Alginates in biomedical applicationsPharmaceutical industries use purified alginates as stabilizer in solution and dispersion of solid substances. In the biomedical domain, alginates are used for controlled drug release13, cells encapsulation14, scaffolds in ligament and tendon tissue engineering or to prepare moulds in dentistry (in presence of slow release calcium salt)15–17.
Alginates in packagingCooking or warming breaded foods in a microwave oven causes a lack of crunchiness. New products are now proposed for microwave heating by using active packaging (with susceptors). In that view, a film of alginate gel with high salt concentration was used as an edible susceptor. Better heating and shorter cooking times were demonstrated18. Edible films were also prepared as emulsifiable alginate with new barrier properties and good mechanical properties also allowing protecting encapsulated active substances19. Incorporation of garlic oil, a natural antibacterial agent gives good edible film20.
Vegetale and fruit preservation was favoured by incorporation of stable silver nanoparticles in alginate film21. Antibacterial films were obtained by incorporation of extruded white ginseng to ensure healthy foods22. Antimicrobial packaging with lactic acid bacteria incorporated in alginate film matrix allows to control the growth of food-borne pathogens in ready-to-eat food23. Antiradical properties were obtained with oregano-based film made of polycaprolactone/alginate-calcium film24.
Alginates in paper industryAlginate partially complexed with calcium (such as forming a loose gel) mixed with starch was proposed to get high water retention in paper coating. Such advantage is important for size press coating formulation to improve coating rheology, control migration and get coat weight uniformity25.
Alginates in textiles and medical textilesA review was recently available which is devoted to processing of alginate fibers for their use as a wound management material26–27. Wet-spinning is the usual way to produce fibers which may be highly absorbent wound dressing materials; especially, mixed sodium and calcium fibers may be produced, calcium giving the wet integrity and sodium the large absorbency. Alginic acid fibers or silver fibers may also be prepared to enhance anti-microbial properties, zinc fibers to generate immune-modulatory and anti-microbial effects. Due to the process in aqueous and mild conditions, it is also easy to prepare fibers for enzyme immobilization or as support for bioactive molecules28.
Alginate calcium fibers were prepared through wet spinning with good tensile strength which can be used for cloth materials. Blending yarns and textile of alginate calcium fibers give good hand feeling and strength. Alginate calcium fibers is an inherent flame-retardant fiber.
Nanoparticles of sodium alginate and 3-(trimethoxysilyl) propyl-octadecyl-dimethylammonium chloride (TSA) with an average size 99nm were loaded onto cotton fabrics by paddry-cure method. Fibers have an efficient antimicrobial activity maintained after 30 laundry cycles (non-leaching antimicrobial agent)29.
Alginates in hemostatic material and wound dressingNew generation of medical textiles are an important growing field, showing great expansion, in wound management products30. The main qualities of fibers and dressings as wound care products include that they are bacteriostatic, anti-viral, fungistatic, non-toxic, high absorbent, non-allergic, breathable, haemostatic, biocompatible, and manipulatable to incorporate medications, with good mechanical properties. Textile structures used for modern wound dressings are of large variety: sliver, yarn, woven, non-woven, knitted, crochet, braided, composite materials. Specialized additives with special functions can be introduced in advanced wound dressings with the aim to absorb odors, provide strong antibacterial properties, smooth pain and relieve irritation. Because of unique properties as high surface area to volume ratio, nanoscale fiber diameter, porosity, light weight, nanofibres are used in wound care. Nanofibers of alginates were produced by electrospinning technique in presence of various synthetic polymers and/or surfactants to improve processability31. Calcium alginate fibers have a novel gel-forming capability in that, upon the ion exchange between sodium ions in the contact solution and calcium ions in the fiber, the fiber slowly transforms into a fibrous gel. The gel-forming process for alginate fibers and the gelling behavior of various types of alginate fibers were described.
The absorption characteristics of alginate wound dressings were analyzed and it was found that alginate wound dressings absorb a large quantity of liquid into the fiber structure, in addition to those held between the fibers in the textile structure. This gives rise to the unique gel blocking properties of alginate wound dressings. In addition, alginate wound dressings also have novel hemostatic and antimicrobial properties as well as the ability to promote wound healing. They are now widely used in the management of highly exuding wounds such as leg ulcers, pressure sores, and surgical wounds28.
Haemostasis and tissue repair is an important domain of development of alginate produced as thin filament in the calcium form. In contact with blood, Ca+2 ions are liberated activating platelet aggregation and reducing coagulation time. Materials are commercialised under different trademarks; as example are: Coalgan from Brothier Laboratories (France) soled as an haemostatic fibers pad for nosebleeds, Algosteril® or Sorban® are also non-woven dressing absorbing rapidly and retains wound fluid resulting in haemostasis and accelerates wound healing32,33. Alginates fibers were reinforced by introduction of hydrolysed chitosans (which diffuse into the alginate fiber) allowing to introduce antibacterial properties34.
ConclusionFrom this short review, it seems clear that alginates are new industrial polysaccharides which may be used in many other applications than only food industry. Nevertheless, biomedical and pharmaceutical applications of purified alginates seem certainely the most promising development of alginates at present time. At end, it is important to mention the recent synthesis of well defined new biohybrid glycopolymers having guluronic oligomers grafted on a hydrophilic backbone giving also gels in the presence of calcium counterions. For many applications, these copolymers may compete directly with alginates35.