To compare effectiveness of OxOral® versus sodium hypochlorite in Enterococcus faecalis elimination at 15 and 60 seconds.
Material and methodsMaterial used in the study was 36 E. faecalis ATCC 29212 cultures assigned to two groups: OxOral® and 5.25% sodium hypochlorite. Both groups were in turn divided into 15 and 60 second samples. Samples were placed in peptone water, 1 mL of irrigating solution and 1 mL of strain were left to rest. 1 mL was extracted at each time, samples were seeded into blood agar for 24 hours. Mann-Whitney U test was applied.
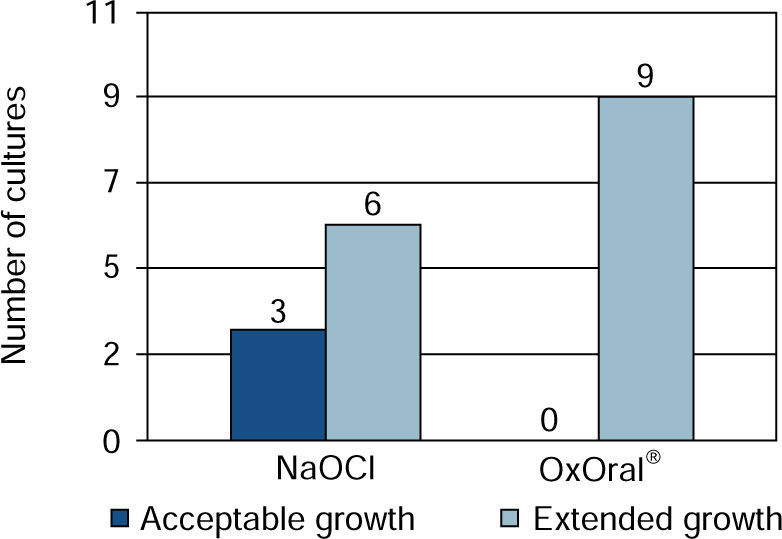
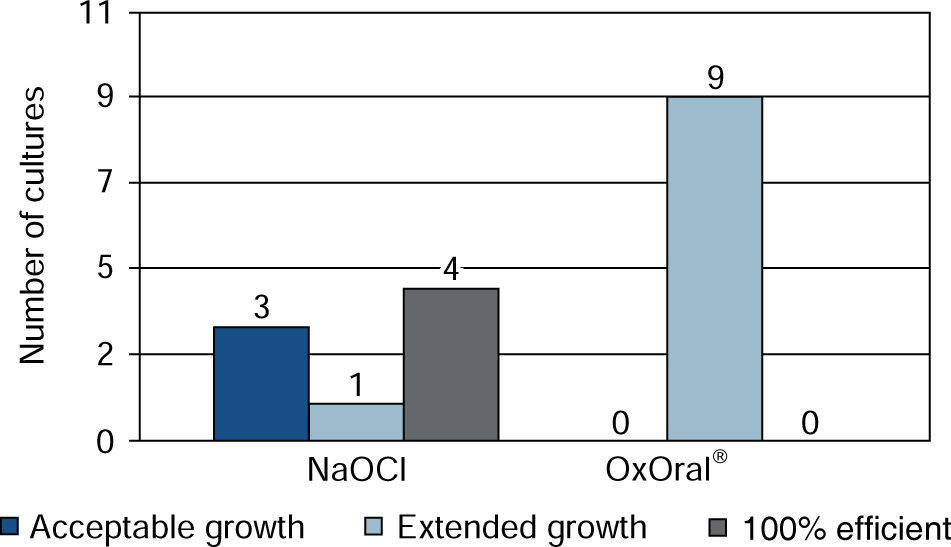
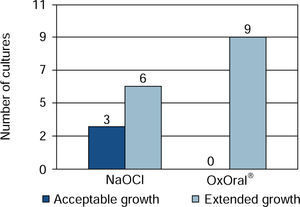
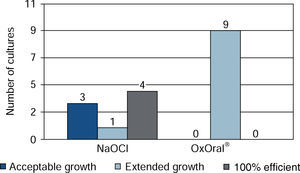
ResultsWith sodium hypochlorite at 15 seconds, there were three cultures with acceptable growth and six with extended growth; at 60 seconds four cultures exhibited effective result, three acceptable and one extended. With OxOral® there was extended growth in all nine cultures at both established times, significant statistical differences were found at the 60 seconds time (p < 0.01).
ConclusionE. faecalis elimination was better with sodium hypochlorite at 60 seconds.
Comparar la eficacia en la eliminación de Enterococcus faecalis con OxOral® versus hipoclorito de sodio a los 15 y 60 segundos.
Material y métodosSe incluyeron 36 cultivos de E. faecalis ATCC 29212 asignados en dos grupos; OxOral® e hipoclorito de sodio al 5.25% que a su vez fueron divididos en 15 y 60 segundos. Se colocaron 8 mL de agua peptonada, 1 mL del irrigante y 1 mL de la cepa, se dejó reposar. A cada tiempo se extrajo 1 mL y se sembró en agar sangre por 24 horas. Se empleó U de Mann-Whitney.
ResultadosCon hipoclorito de sodio a 15 segundos hubo tres cultivos con crecimiento aceptable y seis extendido; a los 60 segundos, cuatro tuvieron resultado eficaz, tres aceptable, uno extendido. Con OxOral® hubo crecimiento extendido en los nueve cultivos, en ambos tiempos, encontrando diferencias estadísticamente significativas a los 60 segundos (p < 0.01).
ConclusiónLa eliminación de E. faecalis fue mejor con hipoclorito de sodio a los 60 segundos.
Root canal treatment targets elimination of injured pulp tissue, bacteriae and their endotoxins. To achieve this the following is needed: irrigation, biomechanical preparation (facilitating disposal of organic tissue) and sealing of root canals so as to prevent their subsequent contamination.1–4 Although biomechanical preparation significantly reduces microbiota, it doesn’t fully eliminate bacteriae in lateral canals, accessory canals, isthmus and apical delta; therefore selection of irrigating solution and medication to use within the canal will be of the utmost importance in order to reach areas not accessible during instrumentation; suitable apical sealing is equally important.5–8
One of the main causes of canal treatment failure is persistent multiplication and migration of bacteriae within the canals toward tissues around the root, caused by deficient chemical and mechanical preparation. Adherence to dentin is the first step for bacterial colonization; later, invasion towards dentin tubules and biofilm formation take place.9,10Enterococcus faecalis is within the range of microorganisms that can be found, this strain is found in 33% of all cases requiring a second root canal treatment,8 with peri-radicular lesions that were not repaired.5 This bacteria has additionally been associated to caries lesions, chronic periodontitis and persisting apical periodontitis.11,12 It possesses the ability to adapt to different circumstances (nutrient shortages, acidity, heat, alkalinity, ultraviolet light), thus it can effectively remain in medicated canals.11,13
Its gelatinase activity contributes to its long term survival within filled canals13,14 favoring bonding with dentin; irrigating solutions contribute to eliminate bacteriae located in dentin canals or tubules.15
There are many types of irrigating solutions available in the market. Such is the case of sodium hypochlorite (NaOCl) hydrogen peroxide, chlorhexidine, EDTA (ethylenediaminetetraacetic acid) and superoxidation electrolyzed solution (OxOral®). Sodium hypochlorite, at concentrations of 0.5 to 5.25% possesses the capacity of eliminating organic residues in areas that instruments cannot reach, it therefore constitutes a suitable anti-microbial agent3,8,16 with tissue dissolution ability.6,7 One of its disadvantages is its toxicity on tissues surrounding the root, it can elicit pain, bleeding, volume increase, inflammation and tissue necrosis.7
Superoxidation electrolyzed solution (OxOral®) exerts disinfectant and sterilizing activity; this is due to its activity on bacteriae, viruses, fungi, spores and its low toxicity on tissues.17 These are electrochemically processed solutions, made from pure water and salt, which induce formation of elements derived from oxygen, hydrogen and chlorine, they are purified through inverse osmosis incorporating sodium chloride under voltage and current parameters to obtain ions and free radicals.17–20Among its antimicrobial properties we can mention, among others, activity against Enterococcus faecalis.17,18 Due to its recent appearance in the market, there are yet few literature reports on its bactericidal effect, existing reports show nil effect on E. faecalis.21
Comparison between super oxidant electrolyzed solution (OxOral ®) and NaOCl has not been widely established; no precise benefits have been demonstrated in order to stop replication of microorganisms and in lesser time of use.
The target of the present study was to compare effectiveness of two irrigating solutions: super oxidant electrolyze solution (OxOral®) and NaOCl in the elimination of E. faecalis at two different times for each irrigating solution (15 and 60 seconds).
Material and methodsAn in vitro experimental study was conducted at the School of Dentistry of the Technological University of Mexico in the timeframe of August-December 2014. The study comprised 36 culture samples of Enterococcus faecalis TCC 29212, assigned to two groups: one for OxOral® and the other to 5.25% sodium hypochlorite; samples were studied at two different times: 15 and 60 seconds.
For recuperation and confirmation purposes, the strain was inoculated in a Petri dish with ram's blood agar at 5%, sowing in stretches for 24 hours at 37.5 oC. Once strain growth was obtained, a well isolated colony was harvested with a sterile handle, touching its upper section; it was then transferred to a test tube with 10 mL peptone water. 1 mL of aliquot was placed. In another test tube with 9 mL peptone water; this process was repeated until obtaining a second tube with lesser turbidity. One ml aliquot of this second tube was taken to be then mixed with 8 mL peptone water and 1 mL disinfectant. It was shaken and then left to rest for 15 and 60 seconds for both disinfectant solutions respectively.
At both times, 1 mL was extracted and seeded in Petri dish by extension in ram blood agar at 5%, and left to incubate for 24 hours at 37.5 oC.
After this, colony forming units (CFU) were counted. Boxes with zero to one colony indicated disinfectant maximum effectiveness. E. faecalis elimination was determined as acceptable (CFU still controlled by the disinfectant from 2 to 100) extended (uncontrolled CFU growth ; over 100) and effective (no CFU). Information was analyzed with program SPSS 17.0; irrigating solution effects were compared with Mann-Whitney U test.
ResultsIt was observed that at 15 seconds, with OxOral® there was extended growth in all nine cultures; with sodium hypochlorite, acceptable growth was observed in three cultures and extended growth was seen in six cultures (Figure 1).
At 60 seconds extended growth was observed in all cultures with OxOral®; with sodium hypochlorite, effective result was found in four cultures, three of them with acceptable growth, one with extended growth and one culture could not be assessed due to processing error (Figure 2).
No statistically significant differences were found at 15 seconds (p = 0.065), it can thus be concluded that no significant bactericidal ability to eliminate Enterococcus faecalis was exhibited by NaOCl and OxOral® when used for 15 seconds. Nevertheless, when used for 60 seconds statistical significant differences were found (p < 0.01) (the case with non evaluated sample was eliminated from the analysis). In the elimination process of E. faecalis, NaOCl at 5% proved to be the most effective when used for 60 seconds.
DiscussionThere are many studies reporting effectiveness of hypochlorite (2.5 to 6%), for this reason, it is used as a comparison point with other irrigating solutions that appear in the market, such is the case of OxOral® whose effectiveness has not been fully studied.
Cobankara7 reports on 5.25 NaOCl effectiveness and chlorine dioxide (ClO 2) in organic tissue dissolution, nevertheless, bacterial content was not analyzed; Wang16 reported that 6% NaOCl exhibited strongest antibacterial activity. In the present study, use of 5.25% NaOCl for 15 seconds revealed extended growth of E. faecalis in six out of nine cultures, that is to say at this time, CFU growth was not controlled by the disinfectant. Nevertheless it was found to be more effective when the time was 60 seconds, since only one culture exhibited growth. Results in the present study concur with those of Gutmann6 who mentions that in order to obtain NaOCl effectiveness as bacterial control and tissue dissolution, it is necessary to work with 2.5 to 6% concentrations. This is similar to Harrison's reports22 of a study effected to verify 2.62 and 5.25% NaOCl antimicrobial effectiveness at period ranging from 15 to 120 seconds in cones contaminated with E. faecalis; he mentioned that after 45 seconds at a 5.25% concentration, and after 60 seconds at 2.62% concentration no E. faecalis bacterial growth could be observed. On the other hand, Souza23 analyzed antimicrobial activity of NaOCl at different concentrations (1%, 0.5%, 0.12% and 0.25%) in paper cones previously contaminated with E. faecalis. Results indicated that it was eliminated at 0.5 and 1% concentrations during 15 seconds, this not being the case for the other concentrations.
When OxOral® was left in place for 15 and 60 seconds, CFU extended growth was found in all analyzed cultures, therefore, no CFU growth control could be reported, that is to say it did not exhibit bactericidal or bacteriostatic capacity against E. faecalis. Rojas 21 conducted an in vitro study on contaminated files of teeth inoculated with the strain; in said study he concurred with our results and mentioned that OxOral® effected nil bactericidal effect on E. faecalis after 15 minutes, as well as after a 72 hour period; in cases when it has previously been used he mentioned that this solution was not effective for sterilization of endodontic instruments infected with E. faecalis.
Meanwhile, Zaragoza24 conducted a study to compare antimicrobial effect of OxOral® Sterilizing and ACCUA Aséptic Hp®. To this effect he used strains of S. aureus, S. mutans, L. acidophilus, C. albicans and E. coli and Pseudomona sp. He mentioned that no inhibition was found when using OxOral ®, therefore it can be concluded that the solution did not meet with properties pertaining to a sterilizing agent.
ConclusionIn the present study it was found that 5.25% NaOCl for 60 seconds was the best disinfectant to eliminate E. faecalis. This was not the case for OxOral® which exhibited growth in all cultures.