Facial asymmetry is one of the main findings during clinical practice with a prevalence between 21-85%; this causes both functional and aesthetic problems, and is manifested by an inconsistency in size, shape, or position of craniofacial structures on both sides of the mid-sagittal plane. Its etiology is attributed to hereditary and/ or environmental factors that may be expressed during the fetal, childhood and/or pubertal stage, including unilateral condylar hyperactivity, functional disharmony of the masticatory muscles, dominance of one cerebral hemisphere, plagiocephaly, unilateral craniosynostosis, among others. The time of evolution prior to its detection contributes to the level of expression of the asymmetry. By means of a literature review, the proposal of a differential diagram and the presentation of a case report which includes facial analysis, cephalometric analysis, 3D tomographic reconstruction and findings of nuclear medicine, differential diagrams are suggested as well as a new classification of facial asymmetry. The differential diagnosis between asymmetry of the glenoid fossa and hemimandibular elongation is established, which requires a careful correlation of clinical findings and imaging tests, since both have similar clinical characteristics, but differ in their therapeutic approach.
La asimetría facial es uno de los principales hallazgos durante la práctica clínica con una prevalencia entre el 21-85%; ésta causa problemas tanto funcionales como estéticos, y se manifiesta por la inconsistencia en tamaño, forma o disposición de las estructuras craneofaciales en ambos lados del plano medio sagital. Su etiología se atribuye a factores hereditarios y/o ambientales que se pueden expresar durante el periodo fetal, infantil y/o puberal, incluyendo la hiperactividad condilar unilateral, desarmonía funcional de los músculos de la masticación, dominancia de algún hemisferio cerebral, plagiocefalia, craneosinostosis unilateral, entre otros. Donde el tiempo de evolución previo a su detección contribuye con el nivel de expresión de la asimetría. Por medio de la revisión de literatura, la propuesta de un diagrama diferencial y la presentación de un caso clínico que incluye análisis facial, análisis cefalométrico, reconstrucción tomográfica 3D y hallazgos de medicina nuclear. Se sugieren diagramas diferenciales y una nueva clasificación de asimetría facial. Estableciendo el diagnóstico diferencial entre asimetría de la fosa glenoidea y elongación hemimandibular, que exige una cuidadosa correlación de los hallazgos clínicos e imagenológicos, ya que ambos presentan características clínicas similares, pero difieren en su enfoque terapéutico.
Facial asymmetry is one of the main findings during clinical practice with a prevalence between 21-85%;1 this causes both functional and aesthetic problems2 and manifests itself by an inconsistency in size, shape or position of craniofacial structures on both sides of the midsagittal plane.3
Its etiology is attributed to hereditary and/or environmental factors that may be expressed during the fetal, infantile period, and/or pubertal stage and may include unilateral condylar hyperactivity,3 functional disharmony of the mastication muscles, dominance of one cerebral hemisphere,4 plagiocephaly, unilateral craniosynostosis and other disorders associated with chromosomal genetic and multifactorial anomalies such as 13q deletion, Williams syndrome, among others that have oral manifestations. 5 Time of evolution prior to its detection contributes to the level of expression of the asymmetry.3
In some cases asymmetries may be mild and hardly perceptible, hence they may not require any type of surgical treatment and the facial and skeletal imbalances may be masked through dental compensation, soft tissue compensation or a change head posture.1
Severt and Proffit6 found in a group of patients with facial asymmetry that only 5% of these involved the upper facial third; 36%, the middle third and 75%, the lower third with lateral deviation of the chin.
Alterations in the upper facial third involve the development of the skull, which is believed to be apparently symmetrical; but the presence of an anatomical difference between the right and left sides may be an indicator of some acquired genetic or congenital pathological condition, so a boundary between what is considered to be a nonperceptible asymmetry and a pathological one must be established.7 Craniofacial architecture develops thanks to the interaction between the different bone structures that compose it, which will be modulated by the role of the organs they harbor.8,9 A clear example of this is how some asymmetries in the base of the skull develop due to its relationship with neural structures such as the brain;7 Serjsen et al. (1997),10 found that growth of the base of the skull between 4 and 5 years of age is more intense and decreases with age until growth finishes.
Embryologically neural crest cells (NCC), considered specific migratory cells, whose origin is located in the dorsal part of the neural tube during development, subsequent to their induction, they de-laminate and migrate to different regions of the embryo, where they differentiate into a wide range of cell types, including peripheral neurons, enteric cells, melanocytes and smooth muscle, among others.
In the cranial region,11 they contribute in large part to the formation of cartilage and bone. Facial NCC cooperate extensively in the development of the skeleton of the frontonasal and membranous bones of the skull, while more posterior cranial NCC fill the pharyngeal arches where they form the jaw, the middle ear, the hyoid bone and cartilage.11
Although the initial patterns of segmentation and migration of NCC are fairly preserved among species, the great diversity of craniofacial morphology in vertebrates indicates that cranial subpopulations of NCC are able to generate specific skeletal structures during the complex interaction that occurs between their intrinsic genetic program and extrinsic environmental signals that they may be exposed to during craniofacial morphogenesis.11
Because of this, birth defects are associated with craniofacial malformations. It is increasingly evident that these anomalies may be attributed to defects in the generation, proliferation, migration and differentiation of NCC produced by alterations in the regulation of genes that are crucial for shaping the neural cranial crest by altering the signaling pathways that regulate tissue interactions during development.
On the other hand, alterations of the middle third compromise the mandibular fossa or glenoid fossa, considered as a structural component of bone in the connection of the mandible to the skull, forming the most active functional craniofacial complex: the temporomandibular joint. 12 However, information reported in the literature on the importance of the position of the glenoid fossa and its interrelationship with facial structures in the development of some type of malocclusion is very limited.13
It has been suggested that the spatial correlation between anatomical structures might determine craniofacial conformation,14 which proposes that the type of articulation that exists between the temporal, occipital and parietal bones is a reflection of forces generated during chewing that are distributed through the skull. This indicates that the mandible and the temporal bones affect their position and movement on a reciprocal basis behaving as a unit.13–15
Changes in the position of the glenoid fossa during growth may influence the development of a malocclusion and a facial asymmetry as an expression of the morphological and functional alteration; likewise, the position of the glenoid cavity may be determined by the role of the mandibular condyle as well as by dental position occlusion as a possible modulator of the continuous remodeling of the morphology of the joint.16–18
The most common types of facial asymmetry are those that affect the lower third of the face and the occlusion. They are characterized by changes in the three planes of space with or without lateral deviation of the chin. According to their etiology and time of evolution, they may be considered mild, moderate, or severe.19
Among the possible causes there are:
- 1.
Unilateral condylar hyperplasia.
- 2.
Asymmetric mandibular prognathism.
- 3.
Laterognathia (chronic or congenital muscular torticollis).
- 4.
Functional laterognathia.
- 5.
Craniofacial syndromes (hemifacial microsomia, craniosynostosis, facial clefts among others).
- 6.
Facial trauma (fracture).
- 7.
Infections (otitis media, varicella zoster virus).
- 8.
Tumors (chondroblastoma).
- 9.
Condylar hypoplasia.19
Etiology may involve genetic factors (congenital malformations, hemifacial microsomia, hemifacial atrophy, degenerative diseases of the TMJ), environmental factors (prenatal and postnatal trauma, infections, deficiencies in the blood supply and hypervascularity as well as neurotrophic disturbances), functional factors (occlusal interferences and habits), tumor factors (osteoma, osteochondroma, chondroma) and hormonal factors (endocrine disorders, somatomedin, growth factors) and hereditary.20
Unilateral condylar hyperplasia (UCH) is a self-limiting pathological condition that generates severe facial deformity at the expense of mandibular asymmetries. It is characterized by the excessive and progressive growth of the condyle and may compromise mandibular neck, ramus and body unilaterally and may be accompanied by pain, alteration of the occlusion and joint dysfunction with aesthetic and functional implications.20,21
It is commonly found in patients between the ages of 10 and 25 years in its active form and, after this age, it is found in its inactive form, more like the clinical sequel left by the disease.20,22
A greater prevalence in the female gender has been reported,23–28 although some authors affirm that there is no predilection for sex, race or side.26 Other studies have reported an increased incidence of UCH in the condyle on the right side with a percentage of 57% compared to the condyle of the left hand side with a 43%.20,24
The pathology may be manifested in three ways: hemimandibular hyperplasia (HH), hemimandibular elongation (HE), and a hybrid form between these two types,19,26 each with different clinical and radiographic characteristics. The prevalence rate between HE and the HH is 15:1.22
HH is characterized by a three-dimensional enlargement of one side of the face, with an excessive growth in the condylar head; the height of the ramus is increased creating a unilateral vertical elongation where the mandibular angle on the affected side descends, alveolar supra-eruption occurs with an inclination of the upper maxilla and of the occlusal plane as a compensating effect.19 An ipsilateral open bite may be found or a over-eruption of the maxillary teeth in search of achieving occlusal contact; there is very little deviation of the chin and asymmetry with a decline of the commissure of the affected side is present.22
A condylar head with few changes in its anatomy, but with an elongated and thinned neck characterizes HE; there are no significant changes in the size of the mandibular ramus, but there is inclination of the maxillary plane with the subsequent inclination of the occlusal and commissural plane. There is also deviation of the chin towards the contralateral side and intraorally, the midline deviates towards the unaffected side, negative torque is observed in the contralateral lower posterior teeth, the occlusion is presented with contralateral crossbite while the affected side generates mesial displacement (class III malocclusion of Angle) (Figure 1).26
In type III or hybrid form the HE and HH are developed on the same side, all the characteristics are combined, with descent of the lower edge of the mandible of the affected side and evident deviation of the midline towards the contralateral side. There is also a marked inclination of the maxillary and occlusal plane.27
Early diagnosis is important because treatment modalities differ considerably, according to the affected structures, the age of the patient, the severity of the asymmetry and the active or inactive state of the pathology.20
Within treatment, it is important to define whether the growth center is eliminated in cases in which active condylar hyperplasia is demonstrated, or, on the contrary, to treat the sequelae with orthognathic surgery and/or orthodontic dentoalveolar compensation when the pathology is inactive.21
The aim of this report is to establish the differences in the evaluation of imaging and clinical tests; necessary for the differential diagnosis between hemimandibular elongation, which is the most common form of condylar hyperplasia and glenoid cavity asymmetry that is located within the asymmetries of the upper and middle facial third presenting features of mandibular lateral deviation that compromise the lower third of the face well as the HE does (Figures 2and3).
The abovementioned supposes a new classification of facial asymmetries where asymmetry of the glenoid cavity (ACG), is present within the structural and functional asymmetries that compromise the three facial thirds. The upper third because the roof of the glenoid cavity is part of the floor of the cranial vault, the middle third because it compromises the temporomandibular joint and the lower third because it functionally affects condylar position, triggering mandibular lateral deviation with joint overload in the affected side.
CASE REPORTPatient of 11 years of age, female gender, mestizo ethnic group; who attends a private practice of orthodontics with the purpose of consultation «I see my face and my bite deviated to one side».
There is no relevant medical, allergic and/or surgical history. Only preventive dental treatments have been performed on her. She does not report habits, but at the muscular level the patient was diagnosed with upper crossed syndrome (UCS) characterized by contraction in the pectoral muscles, trapezius, levator scapula and suboccipitals, and inhibition of the intercapsular and deep flexors.
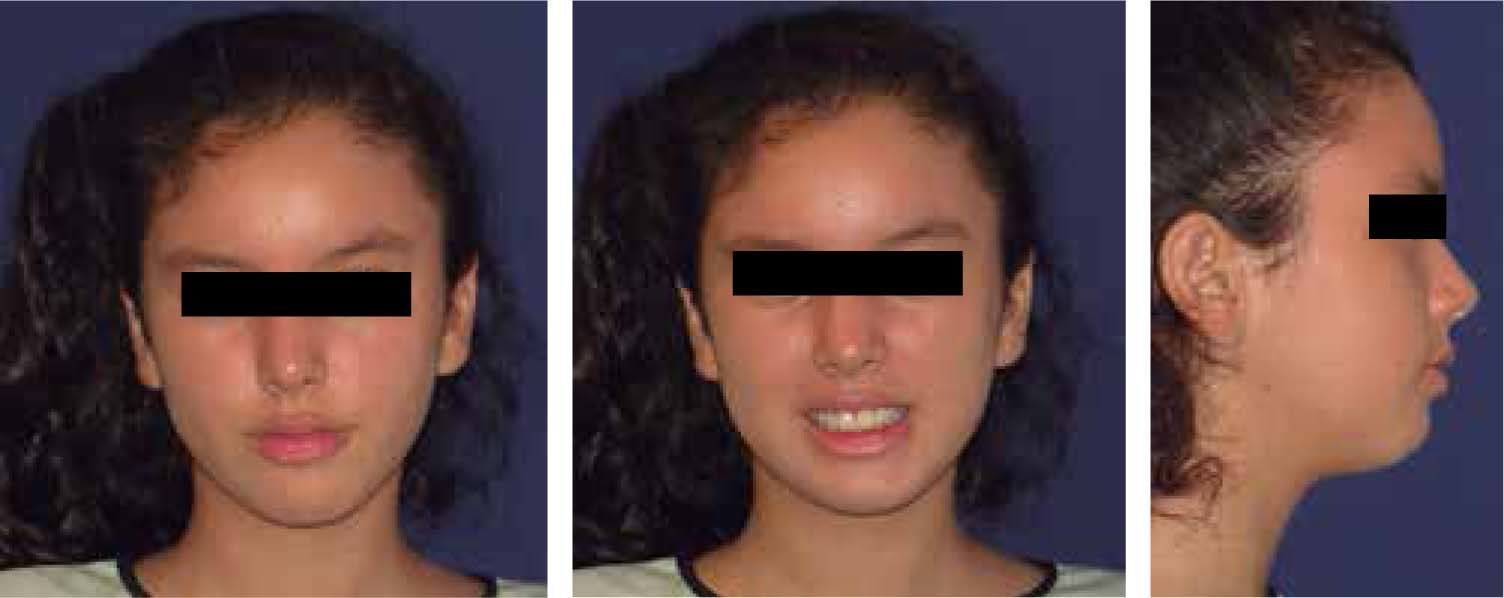
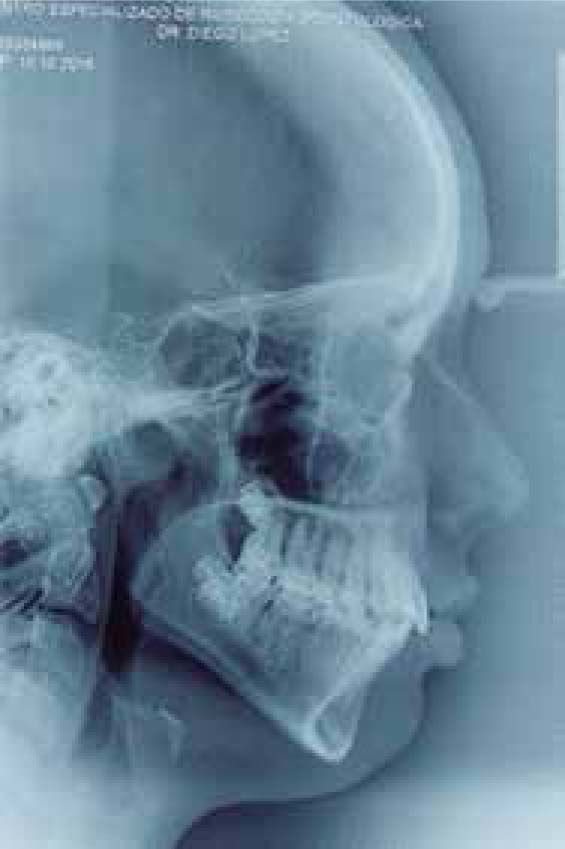
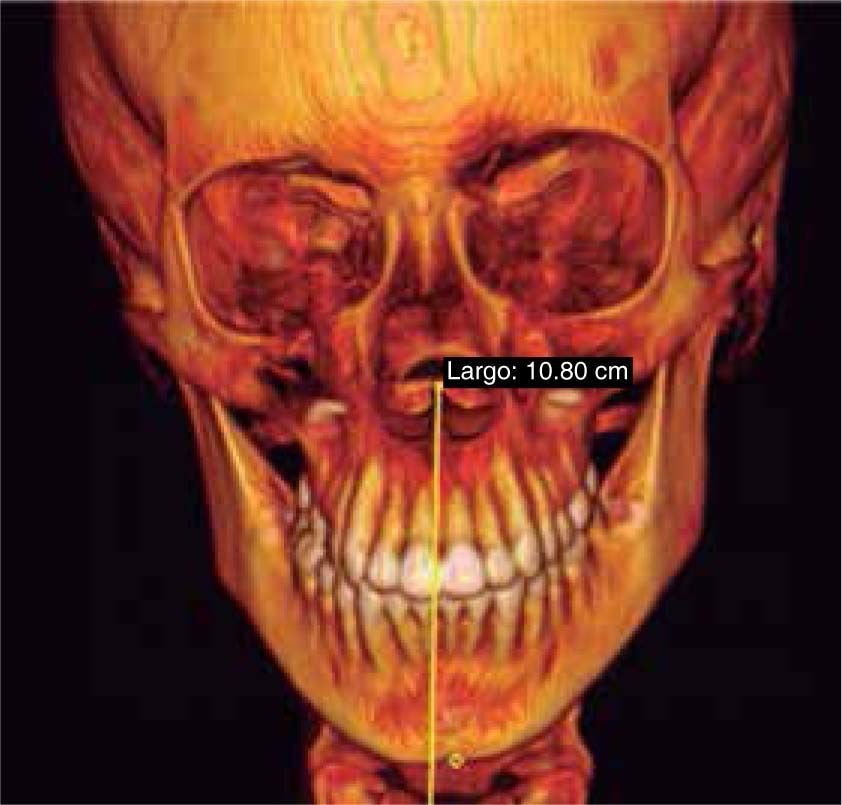
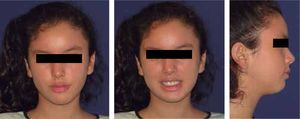
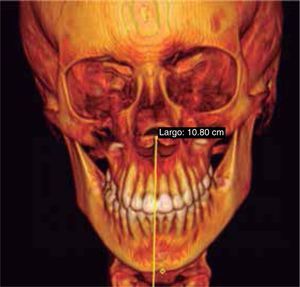
Facial analysisMandibular laterodeviation to the left (mandibular levognatism), asymmetry in the height of the eyebrows and ear implantation, inclination of the occlusal plane and commissural asymmetry, convex profile, oblique forehead, deep mentolabial sulcus, obtuse nasolabial angle, increased lower anterior facial height and postural asymmetry (Figure 4).
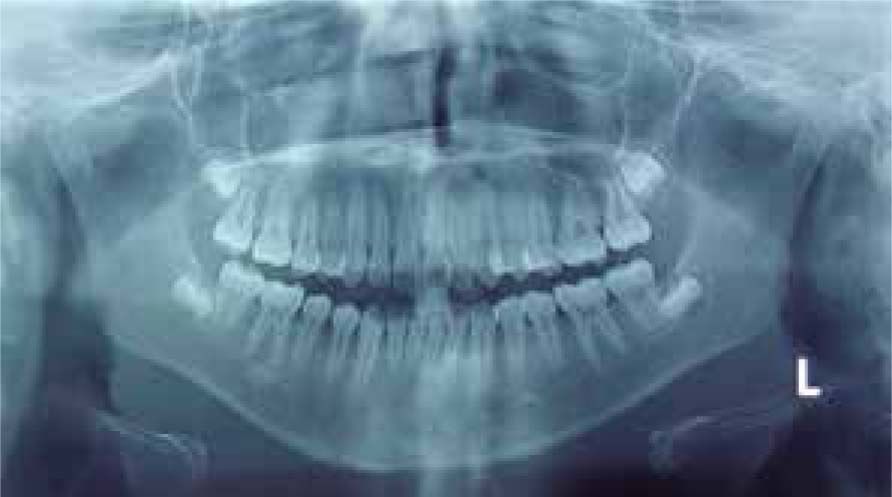
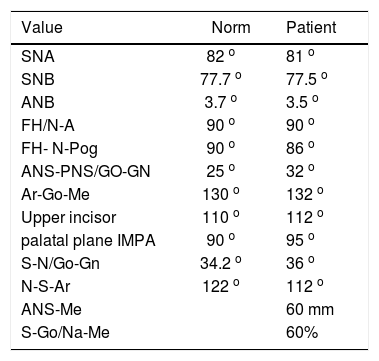
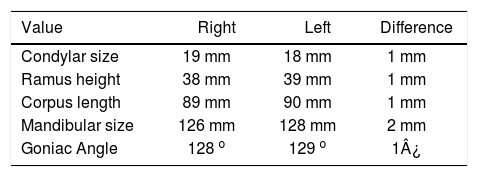
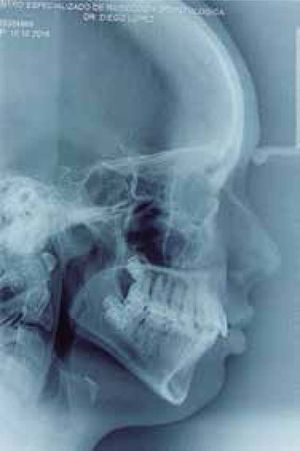
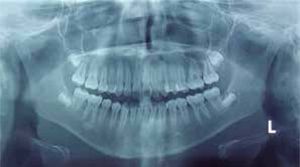
Cephalometric analysisDolichofacial biotype, clockwise mandibular rotation, mild microgenia, mild proclination of lower incisiors (Figure 5andTable I). In the longitudinal analysis of the right and left mandibular anatomy of the panoramic X-ray, no significant differences that induce facial asymmetry were observed (Figure 6andTable II).
Cephalometric values.
| Value | Norm | Patient |
|---|---|---|
| SNA | 82 o | 81 o |
| SNB | 77.7 o | 77.5 o |
| ANB | 3.7 o | 3.5 o |
| FH/N-A | 90 o | 90 o |
| FH- N-Pog | 90 o | 86 o |
| ANS-PNS/GO-GN | 25 o | 32 o |
| Ar-Go-Me | 130 o | 132 o |
| Upper incisor | 110 o | 112 o |
| palatal plane IMPA | 90 o | 95 o |
| S-N/Go-Gn | 34.2 o | 36 o |
| N-S-Ar | 122 o | 112 o |
| ANS-Me | 60 mm | |
| S-Go/Na-Me | 60% |
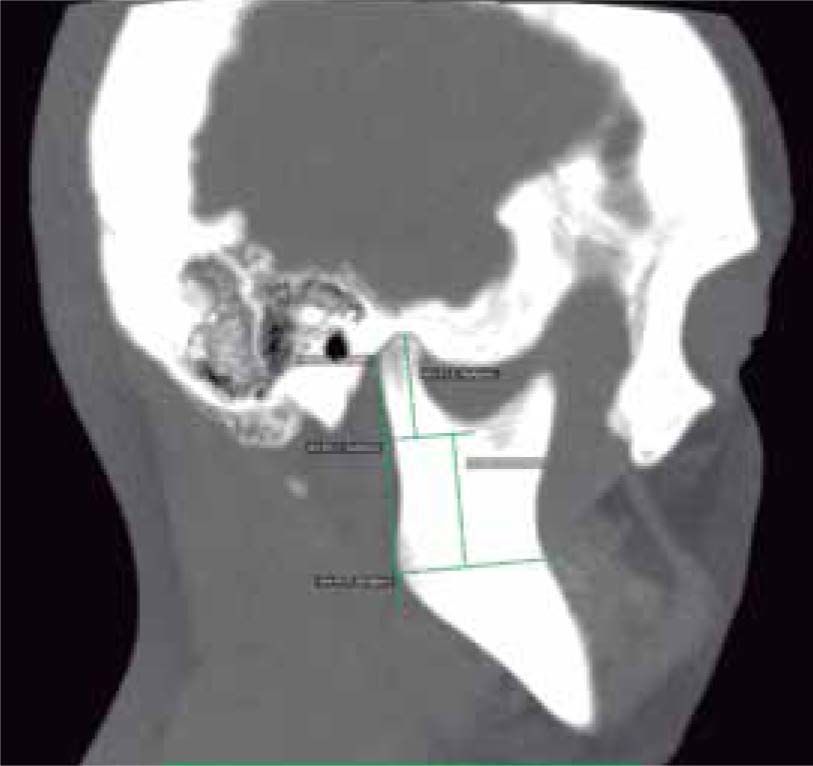
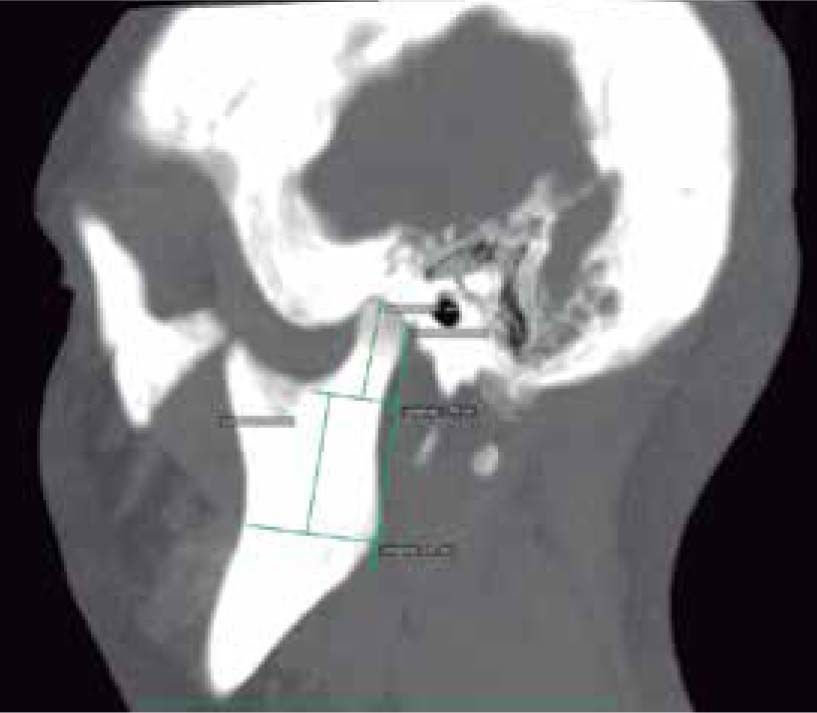
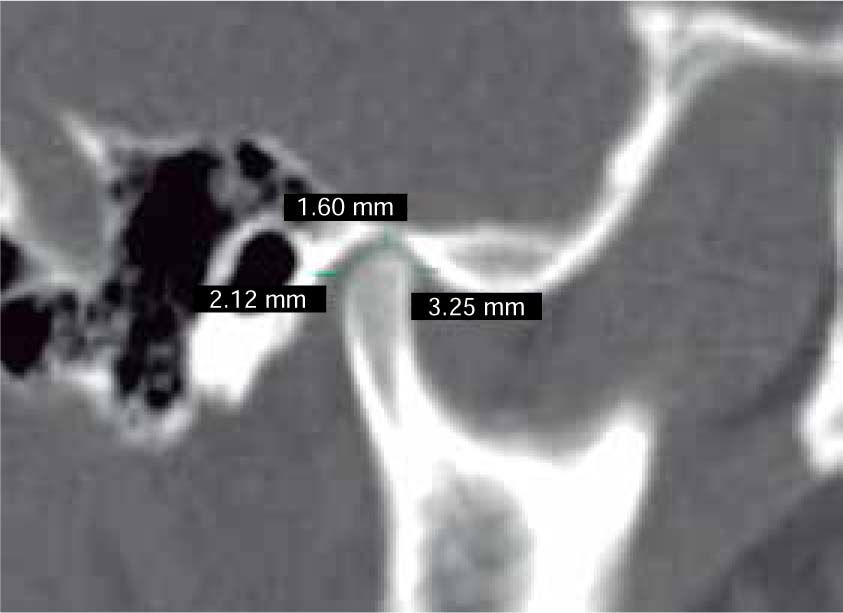
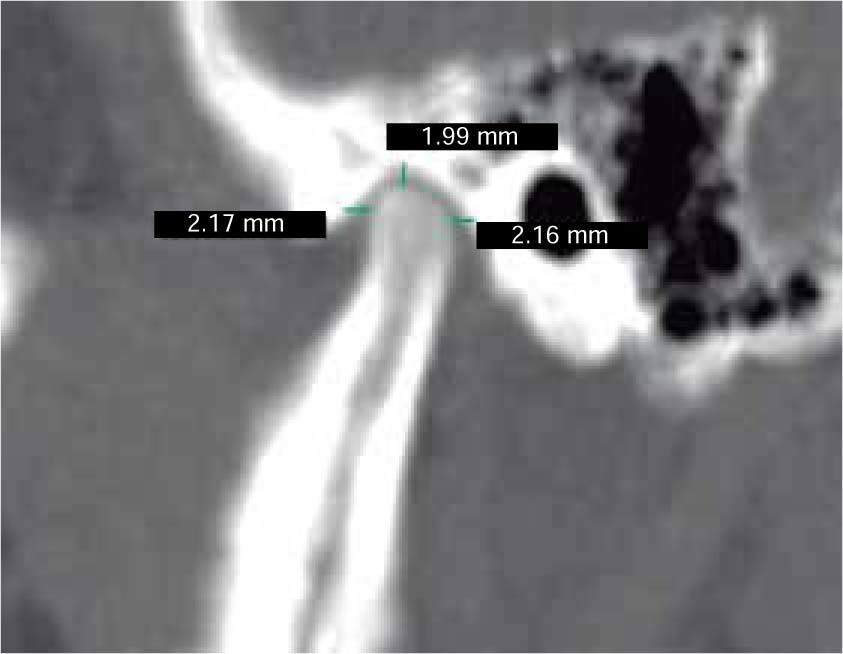
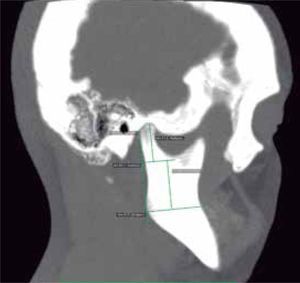
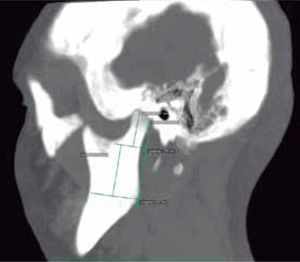
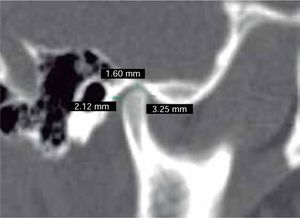
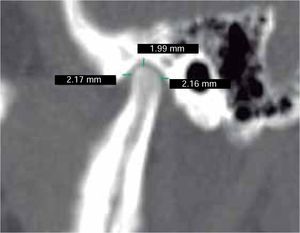
In the sagittal section, condyles and mandibular ramus were observed with discrepancy in measurements of less than 1.5 mm (Figures 7and8). In the same cut, similar intra-articular space measurements were observed (Figures 9and10).
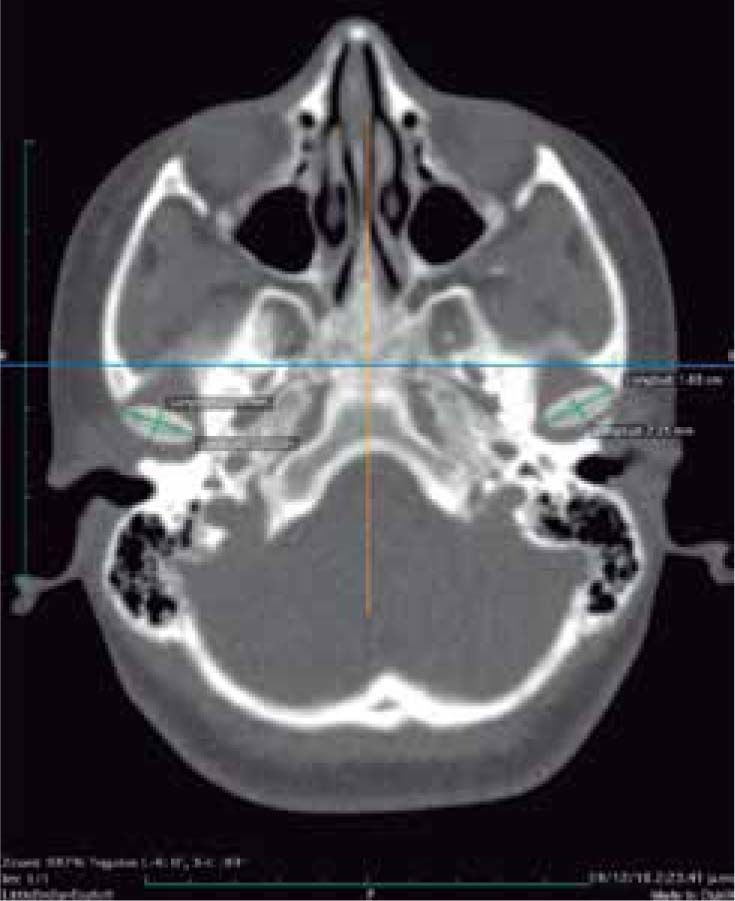
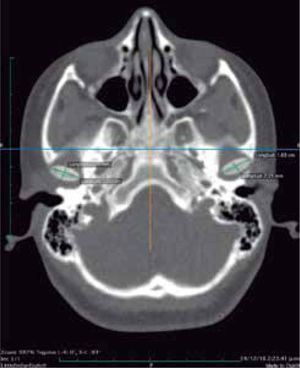
In the tomographic transaxial section, the left mandibular condyle is located sagittally in a more anterior position in relation to the right condyle, showing that the alteration of the glenoid cavity is not only in height, but in three dimensions (Figure 11).
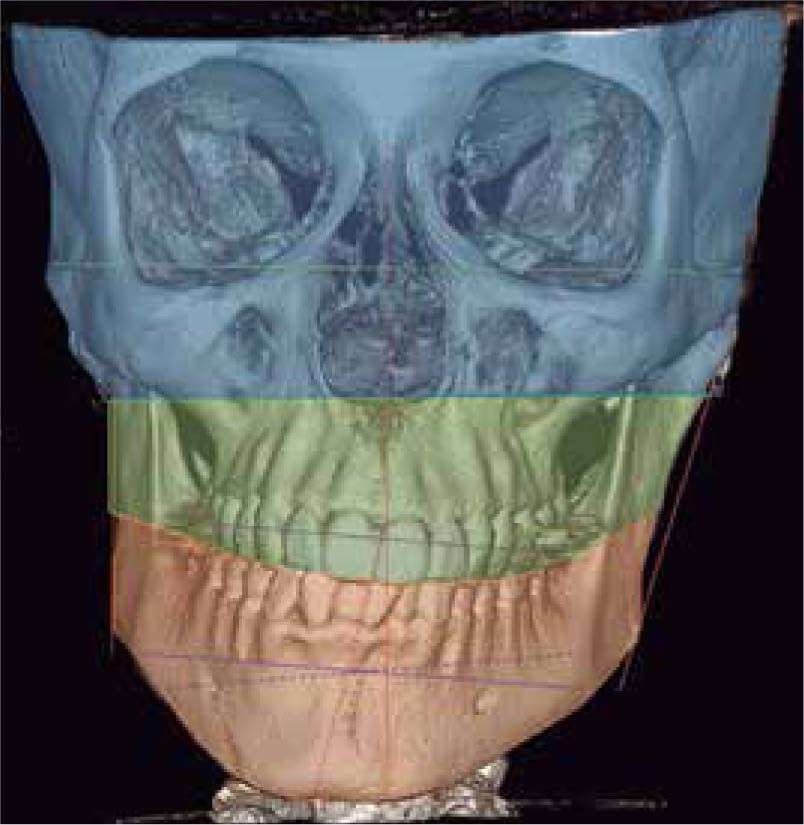
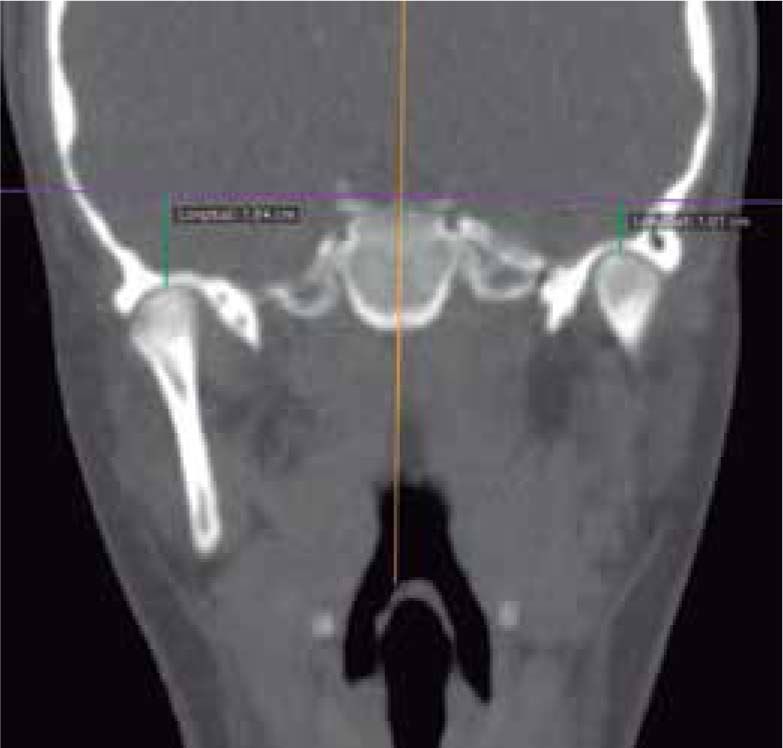
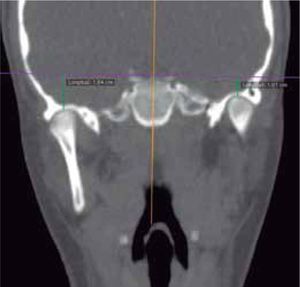
In the coronal section, taking the sella point as anatomical reference, a severe discrepancy is observed between the position of the right glenoid cavity in relation to the left, which is located higher, evidencing the asymmetry of the upper third of the face (Figure 12).
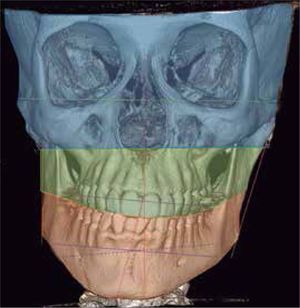
In the images obtained with frontal 3D reconstruction of bone tissues, it is observed that the asymmetry involves structures of the upper, middle and lower third, affecting the position of the orbits, superciliary arch, malar bone, height of the glenoid cavity, external auditory meatus and condyle position (Figure 13).
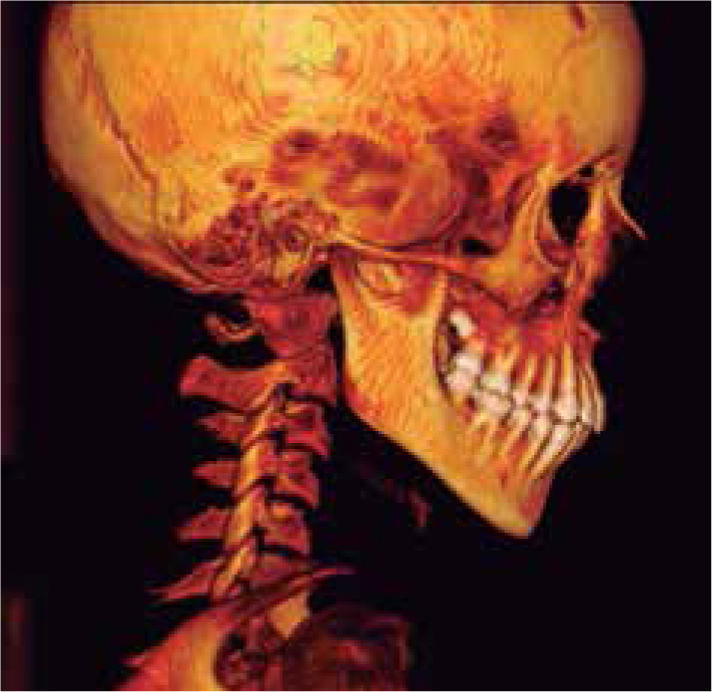
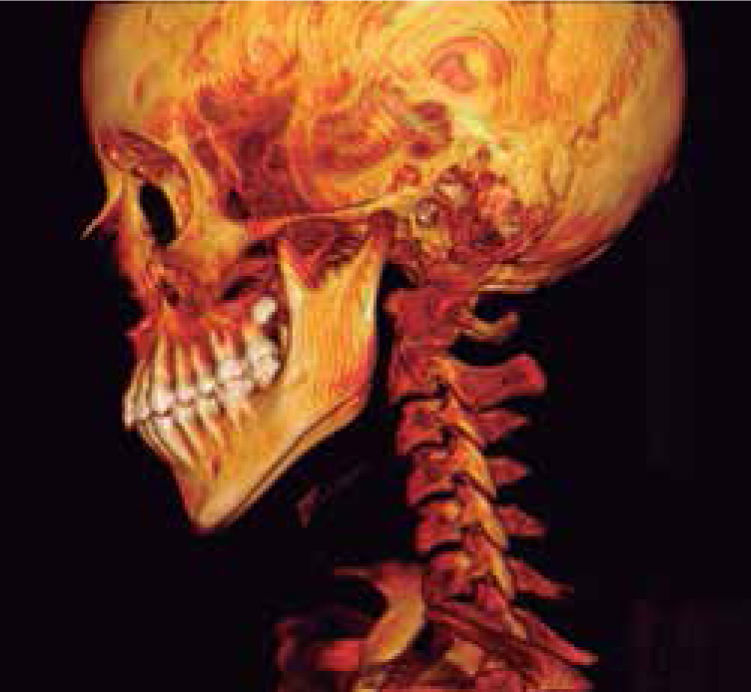
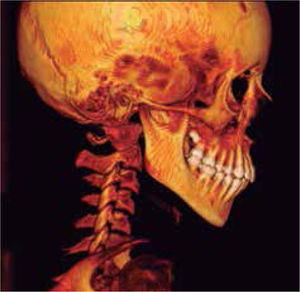
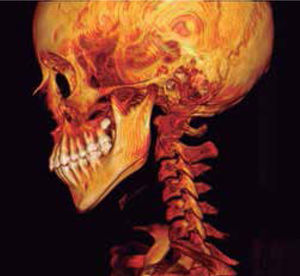
In the left sagittal 3D reconstruction image, a higher position of the left auditory meatus is observed as well as double mandibular bodies product of the mandibular rotation caused by the higher projection of the left condyle inside the glenoid cavity (Figures 14 and 15).
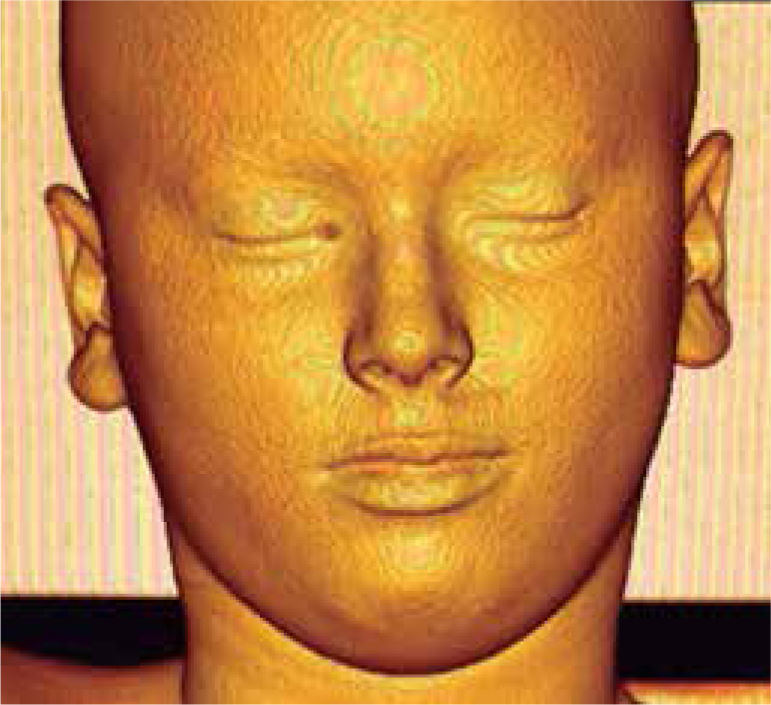
In 3D reconstruction of soft tissues, it is observed a mandibular levognatism compatible with the asymmetry of the middle and upper third that compromises the height of the glenoid cavity (Figures 16and17).
Negative SPECT for left condylar hyperplasia. The radiotracer absorption is similar between both condyles, generating only a difference of 4% in favor of the left condyle, which is within the parameters of normality and suggests a higher uptake possibly by the altered joint mechanics in the left condyle, but not a hyperplasia.
The present case shows how AGC along with its anatomical and structural features compromises the middle facial third producing mandibular lateral deviation and compensations in soft tissues as it occurs with UCH; however UCH a differential diagnosis must be established with other diseases that cause facial asymmetry, such as hemifacial microsomia, hemifacial atrophy, ankylosis, neoplasms, bone tumors, unilateral macrognathism, laterognathia, asymmetric mandibular prognathism and degenerative diseases of the TMJ.20 Although Wolford21 in his most recent classification includes benign and malignant bone tumors as a type of hyperplasia, being a condition that produces excessive growth and elongation of the condyle causing alterations in the bone architecture of the jaw, unlike congenital deformities and endocrine conditions that cause lengthening of the jaw but not as a direct result of the lengthening of the mandibular condyle.22 To this list of alterations that constitute the differential diagnosis of UCH, GCA, which involves the union of the upper third with the middle third of the face with a prevalence of 5 and 36% within the facial asymmetries respectively, should be added.6
Differential diagnosis between these two entities must include their etiological and histological factor as well as pathogenicity of the alteration, age of onset, the ability to produce alterations in time, their extraoral and intraoral clinical characteristics, their imaging characteristics, their results in Nuclear medicine tests and treatment modalities.
HE, which occurs 60% more in women, occurs in adolescence and the pathological process begins generally during the pubertal growth phase, suggesting a hormonal etiology. There is an exaggerated and disproportionate growth of the condyle, developing a progressive facial asymmetry that, due to its self-limiting characteristics, could be active until growth ceases or even continue after it has ended, increasing more and more the sequelae in the three planes of the space.21 While the GCA, product either of defects in the generation, proliferation, migration and differentiation of cranial NCC11 or the modeling of craniofacial architecture from the function of the cerebral organs,7–9 in the first years of life, where the base of the skull is almost completely formed between 4 to 5 years of age10 as well as brain size.28 Therefore, its first signs appear in childhood without a progressive characteristic, but with adaptations and dental and soft tissues compensations that are produced as growth occurs.
From the histopathological point of view, condylar hyperplasia is divided into active and passive (stable situation in which disproportionate growth has ceased). The active state of UCH is characterized by the presence of undifferentiated mesenchymal cells and a layer of hypertrophic cartilage with the presence of chondrocyte islands whose calcification rate appears to be above the normal ranges.29
A characteristic feature of hyperplastic condyles is the distribution of cartilage that rests in the subchondral spongy area and the increase in the thickness of the layers.20,23,30 However, Saridin (2010) 31 and Vásquez (2016) 32 performed a histological analysis of a sample of patients with CH, finding histological differences in condylar architecture, size and definition of the layers,31,32 number of islands of cartilage,31 types of collagen fibers involved,32 and the presence of greater or lesser cellularity;1 highlighting the great variability in the histological presentation of UCH.31,32 The latter produces alterations in cell growth, extracellular matrix production and endochondral ossification for UCH, while for GCA, the alteration is structural in the anatomy of the fossa without changes in the mandibular condyle beyond adaptive variations, product of asymmetric joint mechanics.
Extraoral and intraoral clinical features in both HE and AGC include mandibular lateral deviation with chin displacement, maxillary plane cant, occlusal plane cant, and commissural asymmetry. Dental manifestations may include cross-bite to the contralateral side of the HE or on the side of the AGC; while the ipsilateral class III malocclusion of Angle may be evident in HE12 or in the opposite side to the alteration in the AGC.
It is also important to know all those anomalies of the stomatognathic system that develop in the masticatory muscles, the cranial cervical muscle chain, the ligaments and the temporomandibular joint per se; since these have a direct impact on both posture and postural control. Additionally it has been proposed that alterations produced in the trigeminal afferent nerves could cause an imbalance of the postural chains in the body and affect muscle groups such as the suboccipital and submandibular ones causing compensatory changes in the position of the head, neck and shoulders such as associated cervical hyperlordosis, and thoraxic hypersifosis.33 As described in this case, the patient was diagnosed with an upper crossed syndrome (UCS), which is characterized by a chain-muscle reaction due to the postural imbalance where some muscles shorten and contract, while others are relaxed and inhibited.33
The diagnosis of HD should be made from a correct and meticulous correlation between extraoral clinical characteristics with intraoral and radiographic and/ or tomographic findings. Therefore, in addition to the aforementioned, it is necessary to find anatomical differences in the size, length and anatomy of the condylar neck in two-dimensional images such as panoramic radiographs to correlate them with clinical findings. In this case it was shown that there are no differences in the length or size of the mandibular ramus or condyles that justify the asymmetry. On the contrary, it was necessary to perform a 3D reconstruction tomography that showed differences in the height of the glenoid cavities, as well as a three-dimensional remodeling of the fossa sagittally projecting the left condyle forward. Likewise, the height of the supraorbital arch is greater on the left side as well, such as the malar bone and the position of the external auditory meatus (orbiculo-malar-meatal and articular asymmetry).
Regarding the information provided by SPECT type Bone Scintigraphy, in the case of HE, it is expected that the increased osteoblastic activity in the affected condyle is reflected in the amount of radiopharmaceutical absorption, which for cases of active hyperplasia, shows percentages of absorption equal to or above 55% with respect to the contralateral condyle. Therefore, percentage differences of 10% or more are indicative of active CH. 27,34 In this case, the SPECT-type bone scan showed only 4% differences between condyles with a 52% uptake in the left condyle. These results are within the absorption ranges in healthy condyles that would indicate hyper-uptake, but not hyperplasia.29,34 Therefore, in the case of AGC, the scintigraphy is negative.
Finally, the differences in treatment are related to stopping the active state of the pathology in the case of HE through high condylectomy, in which the condylar articular portion where the growing cartilage is found is surgically removed; or with a low or proportional condylectomy where the total size of the mandibular ramus is normalized to correct the asymmetry.35 In both cases a second surgical procedure or orthodontic correction or compensation of the asymmetry may be needed.36,37
In the case of the AGC there is no corrective treatment of the alteration, only asymmetry compensation procedures. Orthodontic, orthodonticsurgical treatment and physiotherapy are indicated.
CONCLUSIONSAsymmetry of the glenoid cavity, as well as asymmetric mandibular prognathism and the functional laterognasia, should be considered among the differential diagnoses for hemimandibular elongation, which is the most common form of condylar hyperplasia. The affliction of the upper, middle and lower thirds of the craniofacial structures means that its diagnosis is based fundamentally on 3D tomographic reconstruction.