Diabetic nephropathy is the leading cause of end-stage renal disease in Portugal. Previous studies reported that reduction of glycosylated hemoglobin (HbA1c) in patients with Diabetes Mellitus is associated with decreased microvascular complications, including chronic kidney disease. However, few studies reported the relation between HbA1C fluctuations and renal disease. This study aims to evaluate the relationship between fluctuations in HbA1c and renal disease progression in diabetics treated with insulin therapy.
MethodsThis is a retrospective cohort study. Diabetic patients treated with insulin therapy, who were observed between January and December 2011, were enrolled for 3 years follow-up. We calculated the baseline and final follow-up estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration equation and defined patients with or without renal disease progression. Also, we examined the relationship between fluctuations in HbA1c (measured by coefficient of variability) and changes in eGFR.
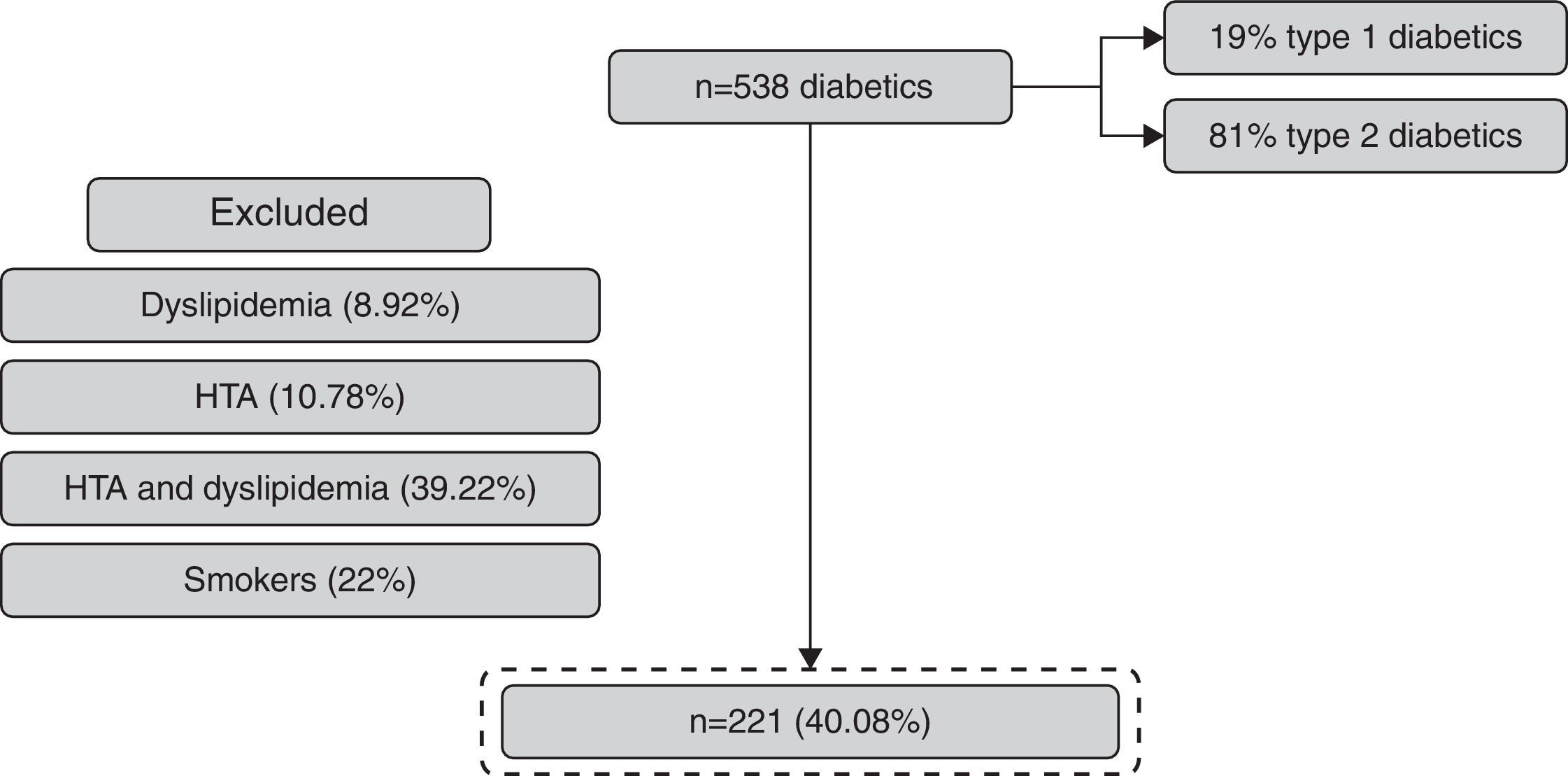
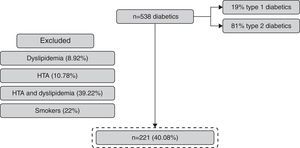
ResultsOf the 538 subjects enrolled, 221 completed the follow-up and were included in the study. 24% had progression in renal status and 76% maintained the eGFR. The coefficient of variability in progression group versus no progression group was 0.0973 versus 0.0812 with p 0.019 (with statistical significance).
ConclusionThese data suggest that fluctuations in glycemic control are associated with kidney disease progression in diabetics treated with insulin therapy.
La nefropatía diabética es la principal causa de lesión renal crónica terminal en Portugal. Estudios previos reportan que la reducción de la hemoglobina glucosilada (HbA1c) en diabéticos está asociada a una disminución de las complicaciones microvasculares, incluyendo la nefropatía. A pesar de esto, pocos estudios relatan la relación entre las fluctuaciones de HbA1c y la enfermedad renal. El objetivo del estudio es evaluar la variabilidad de la HbA1c en diabéticos insulinodependientes e investigar su influencia en la función renal.
MétodosSe procedió a la realización de un estudio retrospectivo, de diabéticos insulinodependientes seguidos en la consulta de Diabetología de un centro de Portugal, de enero a diciembre de 2011, durante 3 años. La población evaluada fue clasificada en 2 grupos: con y sin progresión de la lesión renal, de acuerdo con la fórmula Chronic Kidney Disease Epidemiology Collaboration. Se calculó el coeficiente de variabilidad de la HbA1c, que se define como la razón entre la desviación estándar de la HbA1c intrapersonal y la media de la HbA1c.
ResultadosSe incluyeron 538 diabéticos insulinodependientes de los cuales 221 completaron el seguimiento. El 76% no reflejó progresión de la lesión renal y el 24% sí la reflejó. El coeficiente de variabilidad entre el grupo de progresión y el de no progresión de la lesión renal fue de 0.0973 frente a 0.0812, lo que evidencia resultados estadísticamente significativos siendo p=0.019.
ConclusiónLa variabilidad de la HbA1c influye sobre la progresión de la lesión renal en diabéticos insulinodependientes.
Diabetic nephropathy is the leading cause of end-stage renal disease in Portugal and 30% of diabetic patients develop CKD in all stages.1 Improvements in glycemic control are associated with reductions in the incidence of microvascular complications, including CKD.2
The diabetic nephropathy is a major public health problem worldwide and is associated to other cardiovascular diseases, such as arterial hypertension, dyslipidemia, obesity, coronary heart disease and stroke.3
The concern about the glycemic control to prevent diabetic long-term complications was demonstrated in several prospective studies, which used the mean HbA1c to understand who develop renal disease, more often. Recently, there are some studies with established relationship between fluctuations in HbA1c and its impact in renal function.4 In fact, HbA1c variability is frequent in diabetics and it makes difficult to achieve appropriate glycemic control5.
To our knowledge, this is the first Portuguese based population study, in which HbA1c variability has been used to examine diabetic complications, such as nephropathy, in selected patients treated with insulin therapy (minimizing the impact of oral antidiabetic drugs in renal function), and without arterial hypertension, dyslipidemia or tobacco use.
Diabetic patients with arterial hypertension were excluded because this disease is an important risk factor to proteinuria and glomerulosclerosis.6,7
Dyslipidemia is implicated in atherosclerosis and in cardiovascular complications of renal disease.8 Previous studies show that high levels of cholesterol are related to renal disease progression. Thus we excluded patients with dyslipidemia to avoid bias.
The tobacco is associated with an increased speed in renal disease progression in diabetic and non-diabetic nephropathy because of its vasoconstrictor effect and the increased risk of thromboembolic events.9
The purpose of this study was to examine the relationship between fluctuations in HbA1c over time and renal disease progression in diabetics treated with insulin therapy and without other diseases related to nephropathy.
MethodsThis retrospective cohort study examined adult patients (>18 years old) with established diagnosis of type 1 or type 2 DM treated with insulin therapy, in a medical center in the North of Portugal. The baseline time period was January to December 2011, with a 3 years follow-up.
We measured at least 8 HbA1c values for each patient over the follow-up period (determined by high-performance liquid chromatography) and we collected data of patient's medical records such as age, sex, height, weight, serum creatinine levels (determined with Jaffe's method, calibrated by isotope dilution mass spectrometry) and co-morbidities.
The exclusion criteria included patients with arterial hypertension, dyslipidemia and smokers, to minimize bias in what concerns renal disease progression.
The eGFR at baseline and at last follow-up visit were calculated using the CKD-EPI equation (Fig. 1) and CKD stage was based on NKF criteria.10
Two groups were defined: one with renal disease progression, who had worsened their NKF stage; and the other group without renal disease progression, who maintained their NKF stage during the follow-up period.
The glycemic variability is defined by fluctuation of HbA1c and the coefficient of variability was calculated by the ratio between standard deviation of intrapersonal HbA1c values obtained and the mean HbA1c value.
The statistical analysis was performed by SPSS Statistics® version 20.0, to compare the influence of HbA1c variability in progression and no progression groups, using the Mann–Whitney test, and Chi-square test for categorical variables comparison.
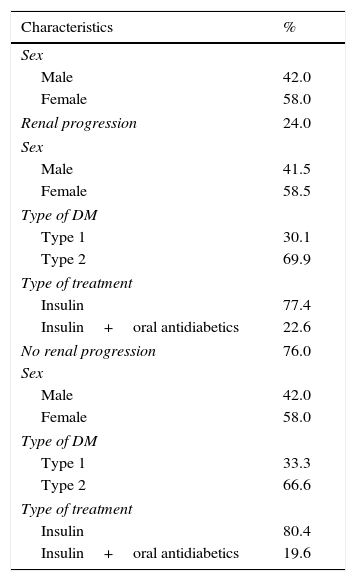
ResultsFrom 538 patients with DM observed, 41.07% (n=221) fulfilled the inclusion criteria (Fig. 2). The mean age of the population was 52±8 years and 58% were women (Table 1).
Patient demographic characteristics.
| Characteristics | % |
|---|---|
| Sex | |
| Male | 42.0 |
| Female | 58.0 |
| Renal progression | 24.0 |
| Sex | |
| Male | 41.5 |
| Female | 58.5 |
| Type of DM | |
| Type 1 | 30.1 |
| Type 2 | 69.9 |
| Type of treatment | |
| Insulin | 77.4 |
| Insulin+oral antidiabetics | 22.6 |
| No renal progression | 76.0 |
| Sex | |
| Male | 42.0 |
| Female | 58.0 |
| Type of DM | |
| Type 1 | 33.3 |
| Type 2 | 66.6 |
| Type of treatment | |
| Insulin | 80.4 |
| Insulin+oral antidiabetics | 19.6 |
BMI > 25 kg/m2 was present in 67% of the population.
The mean number of HbA1c measurements was 8.2±1.5 during the follow-up.
The progression in renal dysfunction was identified in 24% (58.5% were women and 30.1% had type 1 DM) and 76% maintained the eGFR (58% were women and 33.3% had DM type 1).
In the group with maintained eGFR, 19.6% were treated with oral antidiabetic drugs in addition to insulin therapy and, in the progression group, 22.6% were treated with oral antidiabetic drugs; but with the Chi-square test, we obtained p 0.904, which means there are no important differences between these two groups relatively to antidiabetic therapy (Table 2).
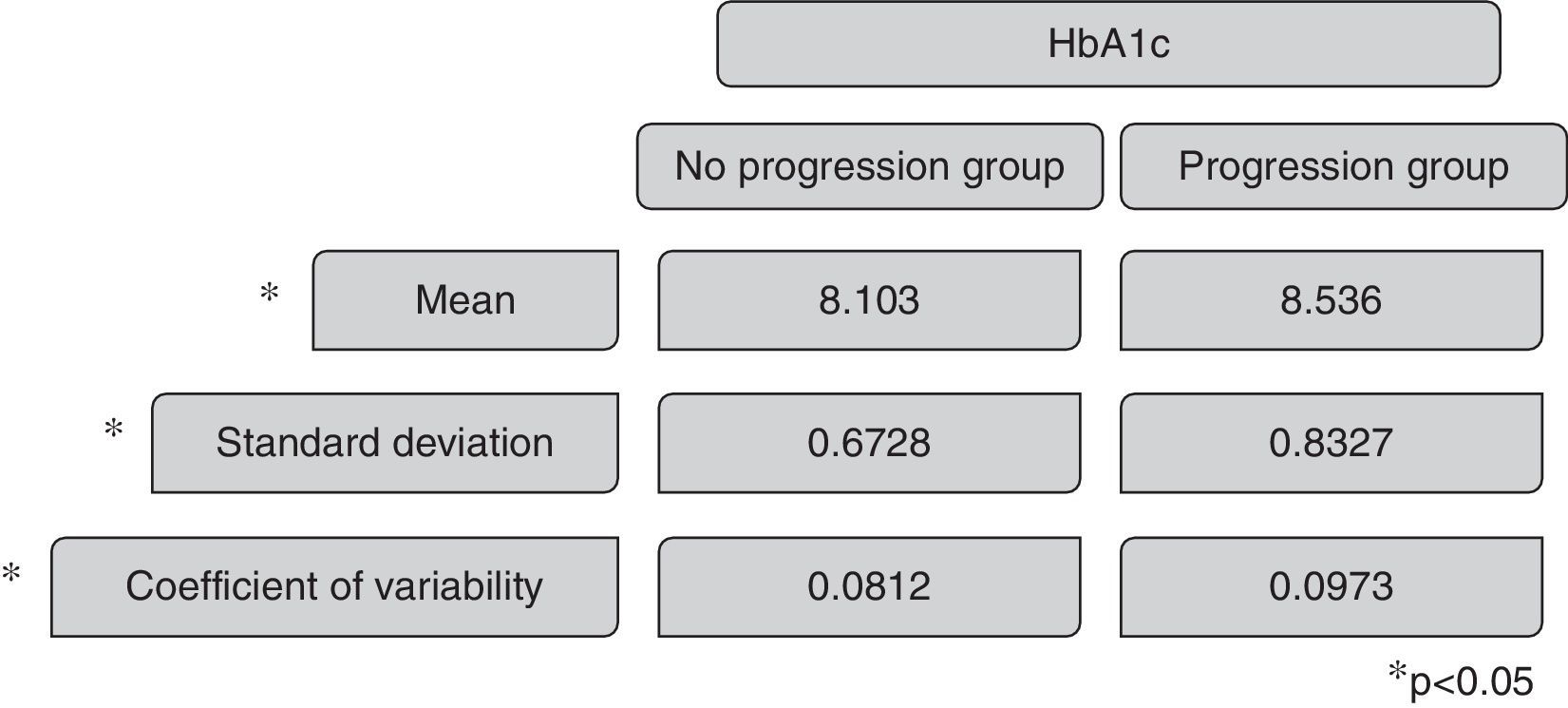
The mean HbA1c in the group without renal disease progression was 8.013, with a standard deviation of 0.7 and a coefficient of variability of 0.0812. The mean HbA1c in the renal disease progression group was 8.638, with a standard deviation of 0.8327 and a coefficient of variability of 0.0978. The Mann–Whitney test revels p 0.019 comparing the coefficient of variability of HbA1c and p 0.033 comparing the mean HbA1c of the two groups, presenting statistical significance (Fig. 3).
DiscussionIt is important to notice the high prevalence of co-morbidities in diabetic patients enrolled in this study. More than 50% of the initial diabetic population had arterial hypertension, dyslipidemia or were also smokers. This emphasizes that the role of diet, exercise and regular follow-up of these patients are crucial to achieve the glycemic control and to prevent or minimize the impact of co-morbidities.11
Importantly, we showed that the two groups have similar characteristics, namely in type of DM and type of drugs used to treat the patients.
Relatively to NKF stages, the progression was more frequent from stage 1 to 2 (49.1%), 1 to 3 (25.2%) and 2 to 3 (12.5%).
We demonstrated that fluctuations in HbA1c are associated with renal disease progression in diabetic patients, which is in accordance with previous studies4,6,12. Also the mean HbA1c has an important role in this subject. These parameters are central in the management of a diabetic patient because of their role to minimize the renal disease progression.
This study has a number of limitations, like the number of patients included and the reduced duration of follow-up. Also, we have not analyzed the duration of DM, which can influence the timing of renal disease worsening.
ConclusionRenal disease progression in diabetic patients is associated with high mean HbA1c and high coefficient of variability of HbA1c, which means that it is important to reduce the fluctuations of HbA1c, in order to minimize the renal disease progression and to prevent the morbidity related to DM complications.13 The fluctuations in glycemic control should be evaluated in the context of the patient's overall risk profile.
FundingNo financial support was provided.
Conflict of interestThe authors have no conflicts of interest to declare.