Surgical treatment for epilepsy comprises resective techniques in most patients.
In those with epilepsy of sites close to highly specialized or eloquent areas, very precise anatomic delimitation is required. So far the most reliable method for anatomic localization of function is direct cortical electrostimulation mapping (CEM). Functional magnetic resonance (fMRI) is a non-invasive method that could also be used for this purpose.
ObjectiveTo determine the sensitivity and specificity of fMRI for the identification of eloquent areas compared to CEM in epilepsy surgery candidates.
MethodsFour patients who underwent presurgical fMRI and grid implantation in eloquent areas for epileptic focus localization with video-EEG were included in this study. Once the seizure onset site was identified, CEM was performed and a postsurgical structural magnetic resonance was reconstructed with Eclipse software to determine grid position. After correlating pre and postsurgical images, the site of the grid contacts where eloquent areas were identified was compared to localization by fMRI.
ResultsOne hundred and twenty electrodes in six eloquent areas were evaluated and compared to location by fMRI: five hand motor areas and one language area in the temporal lobe. We found a global sensitivity of 0.86, specificity of 0.96, positive predictive value of 0.89, negative predictive value of 0.95 and accuracy of 0.94 for fMRI.
ConclusionIn this study fMRI showed high specificity and proved to be useful for language lateralization. It is necessary to study this technique further, especially for language areas.
El tratamiento quirúrgico para Epilepsia comprende técnicas resectivas en la mayoría de los pacientes. En aquellos con epilepsia de sitios cercanos a áreas altamente especializadas (elocuentes o primarias) se requiere una delimitación anatómica muy precisa. Hasta ahora el método más confiable para identificación anatómica de una función es el mapeo cortical directo con estimulación de la corteza cerebral. La Resonancia magnética funcional (fMRI) es un método no invasivo que también pudiera ser usado para este fin.
ObjetivoDeterminar la sensibilidad y especificidad de la fMRI para identificar áreas altamente especializadas en comparación con mapeo cortical por electroestimulación en pacientes candidatos a cirugía de epilepsia.
MétodosEn este estudio se incluyó a cuatro pacientes en los que se efectuó fMRI prequirúrgica e implantación de mallas de electrodos en áreas primarias para registro continuo de video EEG para localización del foco epiléptico. Una vez localizado el sitio de inicio de las crisis, se hizo mapeo cortical por estimulación eléctrica (MCEE) y Resonancia Magnética (RM) estructural que se reconstruyó con el software Eclipse para determinar la posición de la malla. Después de correlacionar las imágenes se comparó la localización de los contactos de la malla donde se identificó el área especializada por mapeo con su localización obtenida por fMRI.
ResultadosSe evaluó la estimulación de 120 electrodos en seis áreas elocuentes: cinco áreas motoras para la mano y un área de lenguaje en lóbulo temporal y se comparó con el área activada en la fMRI. Se encontró para la fMRI una sensibilidad global de 0.86, especificidad de 0.96, VPP de 0.89, VPN de 0.95 y exactitud de 0.94.
ConclusiónEn este estudio la fMRI mostró alta especificidad y gran utilidad para lateralización del lenguaje con aplicación clínica. Es necesario continuar estudiando estas técnicas, en especial en áreas para el lenguaje.
Surgical treatment for epilepsy comprises resective or deep brain stimulation (DBS) techniques. Aspects like focus location and the feasibility of its total resection have great importance. Patients with an epileptic focus far from eloquent areas are candidates for resective surgery with very good results, especially in cases of temporal lobe epilepsy.1
One of the main factors that limit total resection of the epileptic focus is its location in or adjacent to an eloquent area. Patients with multiple foci or those in whom focus resection would cause important sequels require thorough presurgical study since it is very important to correctly identify the eloquent area and how distant it is from the epileptic focus. CEM is the method of choice to predict the functional outcome of epilepsy surgery2,3,4 since it allows precise cortical location of the function being studied. At the Epilepsy Clinic of the General Hospital of Mexico CEM is performed extraoperatory, through grid electrodes, at the telemetry room.
Functional magnetic resonance (fMRI) is a non-invasive method for delimitation of functional areas. It uses the magnetic properties of hemoglobin in order to detect increased blood flow in sites activated by specific tasks. After image processing, this zones are represented colored.
The most used tasks for motor fMRI include consecutive movements of contralateral hand, either two-finger tapping, multiple-finger tapping, opening and closing the hand or squeezing a ball.2,5
For language there are several tasks depending on the specific area of interest. There are image naming tasks or silently thinking of words.2 The most used tasks to determine language dominance are those of generating words of a certain category, mainly verbs, from others represented with images.1,9 Combining different tasks that activate the same area during fMRI acquisition has proved to increase its sensibility.2 Thus, a word generation task combined with a word listening task could better identify language areas than these tasks performed separately.
Reports comparing fMRI and CEM to localize eloquent areas are relatively scarce. Most publications on the matter highlight the fact that fMRI has higher sensibility and specificity for motor areas tan for language areas, and that more studies with a well-defined methodology are required before we can say that fMRI could substitute CEM.2,7,8,10
MethodsThis observational study was approved by the IRB and IEC of the General Hospital of Mexico. Four patients older than 18 years who required presurgical fMRI and grid implantation for epileptic focus localization were included (Table 1). They all signed an Informed Consent Letter.
All fMRIs were acquired with a 1.5T General Electric device. An EPI BOLD 2040 image sequence with 4mm slices, 0mm space between slices, TE of 40, TR of 3000, FOV 25×25, matrix 64×64, 90°, was used. They were then processed at the General Electric work station.
For motor areas the opening and closing the hand task was used. It was repeated three times during the BOLD sequence acquisition time. For language in temporal lobe a noun generation task using images was used. Three series of 10 images were presented to the patient on a screen during the BOLD sequence acquisition time.
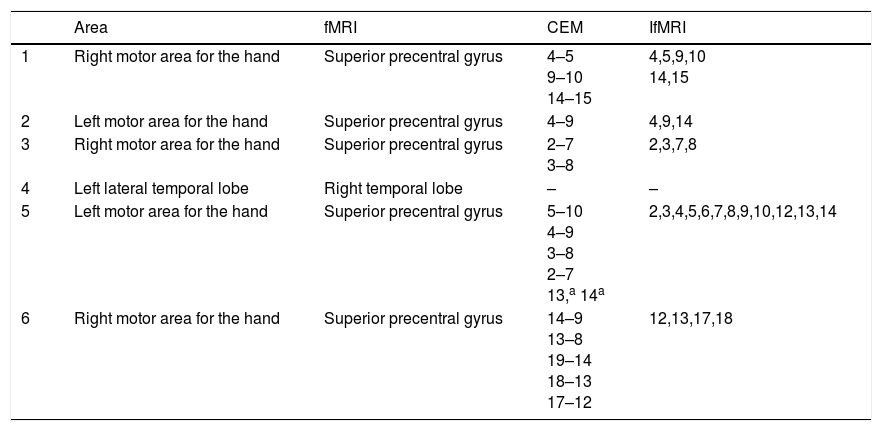
Surgical techniqueFor grid implantation in supplementary motor area and primary motor area a posteriorly based horseshoe shaped incision was performed. A wide craniotomy was made, 2cm to the front and 6cm behind coronal suture, and 5cm lateral to sagital suture on both sides. After durotomy central sulcus was identified and grids were placed. For temporal lobe implantation a Falconer incision and a 5×5 craniotomy were made.
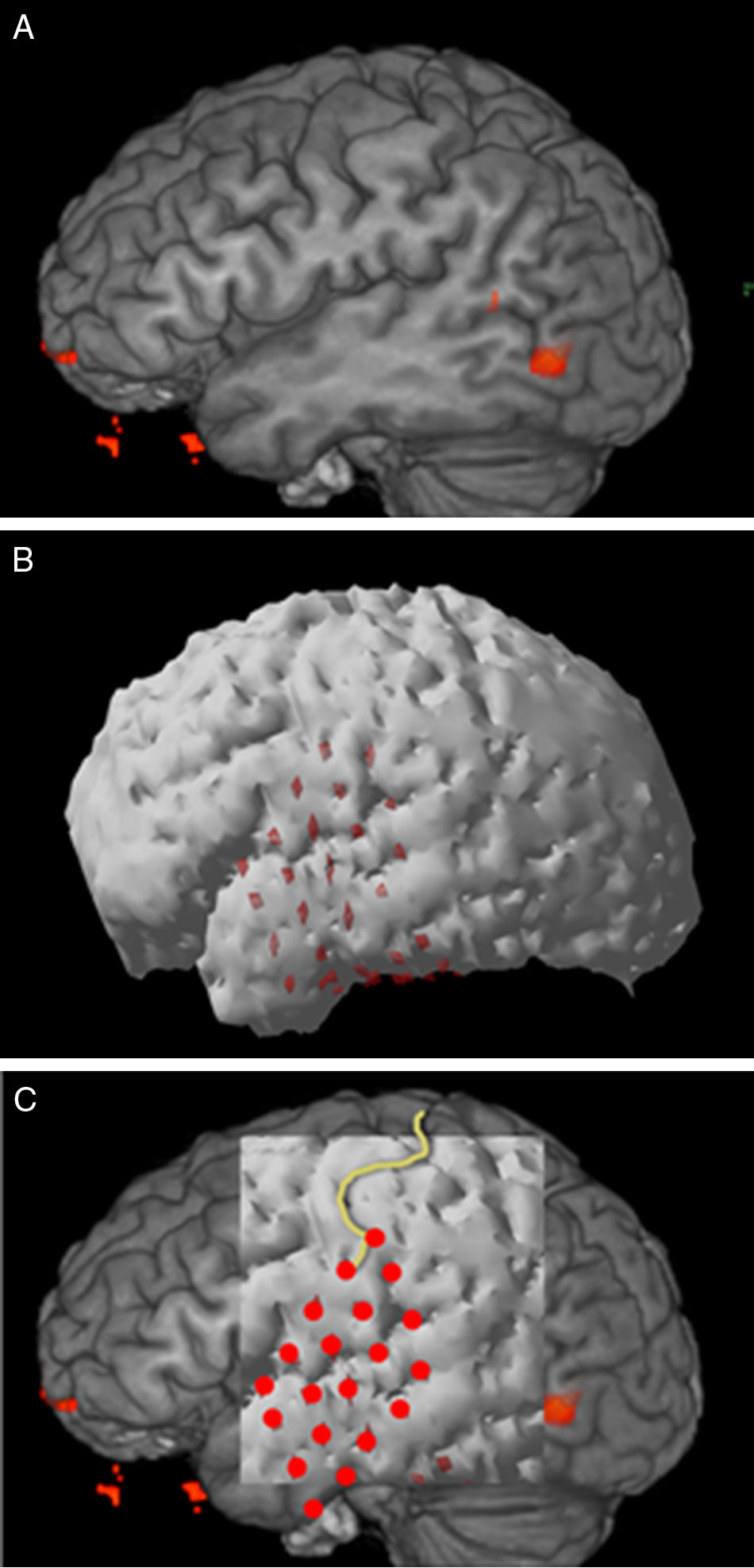
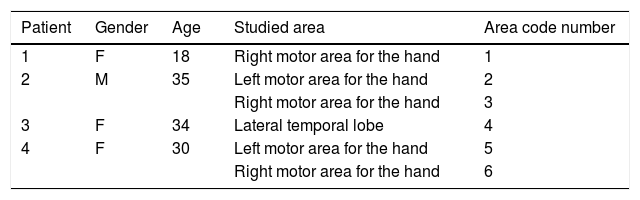
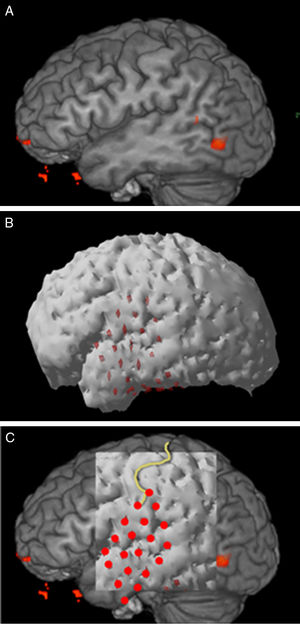
Structural MR 3D reconstructionPostsurgical structural MR reconstruction was acquired with a 1.5T Siemens device. Skull ap and lateral X-rays were performed too. MR was processed with Eclipse software using the sequence where grid contacts were best identified. Fig. 1 shows the process of 3D reconstruction and correlation.
CEMThis was made after the epileptic focus was identified. Direct bipolar stimulation through 20-contact Integra grids was performed. A Grass square-pulse stimulator model S88 was used.
Bipolar stimulation was made in all grid electrodes. CEM was made blindly by the Neurophysiology team. Once the desired response was identified in a given pair of contacts, the test was repeated two more times.
ResultsfMRIThe opening and closing the hand task could show the hand motor area in all cases. In these patients surface EEG showed frontocentral epileptic activity, either unilateral or bilateral, and they clinically displayed hand or arm movement, thus there was a probability that the epileptic focus was found in the hand motor area. The objective in these patients was to identify that area.
In the case of left temporal lobe, during the noun generation task there was no activation of the left side, but of the right one. It was confirmed that the patient had right dominance for language. Clinical, electroencephalographic and image findings were highly suggestive that the epileptic focus was on the left side. So, the most recommended treatment was temporal lobectomy. A dicotic listening test had been made in order to determine language dominance, but results were not clear. In this case, the objective was to rule out function localization in the side where lobectomy would take place.
Video-EEGElectrode grids were implanted in order to identify the epileptic focus with an extraoperatory continuous video-EEG.
In motor area patients grids were placed in primary and supplementary motor areas. Primary motor area grid was used for video-EEG and CEM; the supplementary motor area grid was used only for video-EEG. The epileptic focus was identified in the primary motor area only in one case, in the other patients it was located in the supplementary motor area.
For left temporal lobe, a lateral grid was placed for CEM and video-EEG, and a subtemporal grid was placed for video-EEG. The epileptic focus was identified in the medial basal region, in the anterior parahippocampus. Both fMRI and CEM did not identify language function on this side. Since it was known from the beginning that the epileptic focus was on the left side, a right grid was not implanted and CEM was not performed on this side. On the other hand, being fMRI a non-invasive study, it could evaluate both sides and localize language dominance on the right side. This was corroborated clinically: after left anterior temporal lobectomy there were no language deficiencies in neuropsychological testing.
CEMWe mapped three right motor areas for the hand, two left motor areas for the hand, and one left lateral temporal lobe. In each case a 20-contact grid was used.
In all motor areas for the hand contralateral hand movement was confirmed. Single pulses were used starting with 5V and increasing by 5V until a response was obtained or up to a maximum of 60V. Mean voltage required to produce a motor response was 6.6V. In area 5 there were two contacts that elicited both motor and sensory response.
For language area stimulation was made while the same noun generation task used for fMRI was performed. We used continuous stimulation with a frequency of 60Hz, 4.5μs pulse width, starting with 5V and increasing by 5V until a response was obtained or up to a maximum of 60V. No language impairment was found in any of the contacts.
Table 3 shows the contacts where function was identified in each area.
Structural MR and 3D reconstructionIsometric sequences T1 mpr axial, T2 spc sagital and T2 TSE 3D axial were acquired, and coronal reconstructions of each were made. The best sequence for brain surface definition and grid contact identification with Eclipse software was T1 mpr (Fig. 1).
Image correlationIt was made with the method of anatomic landmarks. Once the correlation was made, the numbers of the contacts where fMRI had identified function were grouped under the variable IfMRI.
fMRI and 3D reconstruction concordanceConcordance was observed macroscopically in all cases (Table 2).
Positive contacts.
| Area | fMRI | CEM | IfMRI | |
|---|---|---|---|---|
| 1 | Right motor area for the hand | Superior precentral gyrus | 4–5 9–10 14–15 | 4,5,9,10 14,15 |
| 2 | Left motor area for the hand | Superior precentral gyrus | 4–9 | 4,9,14 |
| 3 | Right motor area for the hand | Superior precentral gyrus | 2–7 3–8 | 2,3,7,8 |
| 4 | Left lateral temporal lobe | Right temporal lobe | – | – |
| 5 | Left motor area for the hand | Superior precentral gyrus | 5–10 4–9 3–8 2–7 13,a 14a | 2,3,4,5,6,7,8,9,10,12,13,14 |
| 6 | Right motor area for the hand | Superior precentral gyrus | 14–9 13–8 19–14 18–13 17–12 | 12,13,17,18 |
For the diagnostic test analysis, fMRI identification was considered positive in all cases where the colored spot was exactly under a CEM positive contact, with no margin beyond the electrode edge.
fMRI had a sensibility of 0.86, specificity of 0.96, positive predictive value of 0.89, negative predictive value of 0.95 and accuracy of 0.94. On the sites considered as false negative, the mean distance between the colored spot and the grid contact was 7.25mm.
Surgical treatmentFinal treatment is shown in Table 3.
Final treatment.
| Patient | Mapped site | Focus location | Treatment |
|---|---|---|---|
| 1 | Right motor area for the hand | Right motor area for the hand | Cortical stimulation |
| 2 | Left motor area for the hand Right motor area for the hand | Right supplementary motor area | Cortical stimulation |
| 3 | Lateral left temporal lobe | Left hippocampus | Left anterior temporal lobectomy |
| 4 | Left motor area for the hand Right motor area for the hand | Left supplementary motor area | Cortical stimulation |
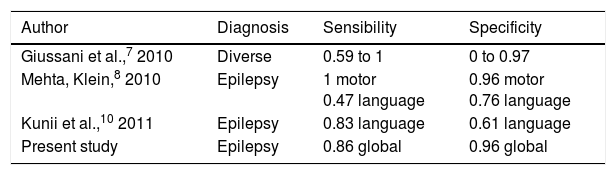
Results obtained for sensibility and specificity are similar to those of previous studies (Table 4).
A separate analysis for motor and language areas was not made because for the latter only one area was evaluated and all contacts were true negative.
Unlike previous studies, we did not intend to identify all cortical areas activated during language production, but only those located at the site where surgical approach is being planned. In patients with temporal lobe epilepsy, resective treatment has excellent results for seizure control, but may have important sequels if performed on the dominant side for language. fMRI showed high specificity, in this study it proved to be a reliable tool in the designing of surgical treatment on the temporal lobe since it correctly identified the eloquent area was not in the usual site. We expected fMRI sensibility to be higher than 0.9. We consider that the fact that it is smaller can be associated mainly to factors regarding CEM and concordance analysis.
With respect to CEM, in motor areas it is practically impossible to reproduce, through electrode pair stimulation, the movement of opening and closing the hand. Response is considered positive when there is sudden contraction of localized muscle groups, which clinically is represented as finger or wrist flexion or extension. It may be due to this difference that a higher sensibility for fMRI was not obtained. For future studies it will be convenient to combine other motor tasks.
As of concordance analysis, in previous studies comparing CEM and fMRI a certain tolerance margin around contacts has been established. Roux et al.6 considered a positive fMRI identification if the colored spot was within a diameter of 1cm from the electrode center. Kunii et al.10 made ROC curves using distances of 3 and 6mm from the electrode edge obtaining best results with the 3mm distance. In this study we decided not to give a tolerance margin.
ConclusionCortical electrostimulation mapping is still the gold standard for eloquent areas delimitation. Nevertheless, fMRI is also a very useful tool for their identification in epilepsy surgery candidates. This study provides more information on the respect and fMRI is still being evaluated in our clinic.
FundingFunding for this study was provided by the Research Department of the General Hospital of Mexico, project number DI/10/403/04/074.
Conflict of interestThe authors declare that they have no conflict of interests.
The authors thank the Department of Medical Physics of the service of Radiotherapy of the General Hospital of Mexico for their support in the development of this study.