Current studies based on specific naming subcategories are scarce and specially if they are combined with cortical electrical stimulation mapping (CES). Most researchers use generic categories of objects that have not shown to be useful for epileptic patients. The present study explores the ability to retrieve words with the aim of naming entities through CES during surgery of a patient with left temporal lobe epilepsy (TLE) prior to anterior temporal lobectomy (ATL). During the CES we observed alterations in the naming of faces, living things (LTh) and parts of the body, with conservation of non-living things (NLTh). CES allowed the identification and prediction of regions at risk of being resected. This result shows that the naming deficits are selective and can be used as indicators and predictors of post-operative cognitive dysfunction in TLE since the regions involved in this function are associated with the pole and inferior and medial temporal gyrus.
Actualmente los estudios de subcategorías en la denominación son escasos y más cuando se combinan con estimulación eléctrica cortical directa (EEC). La mayoría usa objetos que no han mostrado ser específicos en epilepsia del lóbulo temporal (ELT). El presente estudio explora la recuperación de las palabras con el objetivo de nombrar entidades (denominación) a través de la EEC durante la cirugía de una paciente con ELT-izquierdo previo a la lobectomía temporal anterior (LTA) Durante la estimulación se observaron alteraciones en la denominación de rostros, seres vivos (SV) animados y partes del cuerpo, con conservación de los seres no vivos (SNV). La EEC permitió identificar y predecir las regiones con riesgo de ser resecadas. Este resultado muestra que las alteraciones en la denominación son selectivas por lo que pueden emplearse como predictores de disfunción cognitiva postquirúrgica, ya que esta función depende de polo y giro temporal inferior y medial.
TLE occupies 70% of all focal epilepsies and is the most common type of epilepsy in adults and children. Clinical and epidemiological studies have shown that approximately one-third of patients with epilepsy develop epilepsy refractory to pharmacological treatment, despite good administration of prescribed drugs.1
ATL has been described as the most appropriate therapeutic option for to treat refractory TLE, since it is shown to produce a rate of improvement in children and adults between 67.9% and 85%; this rate is superior to any medical treatment.2 The surgical procedure consists of the resection of the anterior temporal pole, anterior part of the parahippocampus, the inferior temporal gyrus and portions of mesial structures such as the amygdala and the hippocampus.3
It has been shown that 81% of patients achieve a favorable outcome for surgery (Class I on the Engel scale) after 6 months of the procedure. The maintenance of the result has been reported in 78% at one year of surgery, 76% at two years, 74% at five years, and 72% at ten years after surgery.4 However, one of the main side effects to surgical treatment is the risk of cognitive alterations, significantly affecting the functionality and quality of life of the patient.5,6 Within these alterations are the loss of episodic memory, language deficits and quadrantanopsis.7 As for the language alterations, it is reported that between 25 and 60% of the patients with left hemispheric dominance submitted to ATL, present a deficit; the difficulties in naming are the highest occurrence ones.8
Naming is defined as the ability to name a conceptual entity through the retrieval of its lexical label (the word that allows naming it). Naming a concept implies access to the lexicon (mental dictionary) and extracting all the information about the words: its written form (spelling), its auditory form (phonology), its meaning (semantic), its morphological structure and its syntactic and semantic category.9 The neuronal substrate of word retrieval does not depend on the classical regions of language (Broca and Wernicke area). Imaging and lesion studies indicate that there are multiple and diverse areas that underlie normal language processing. It is possible to differentiate two systems that work independently. The first one dedicated to the recovery of the lexical label (naming) and the second dedicated to the recovery of conceptual knowledge of objects (recognition).10 It has been described that there is a clear differentiation between brain areas dedicated to word retrieval processing according to the type of category to which the object to be named belong (LT and NLT). Patients with unilateral lesions of the left hemisphere show greater difficulties in the naming of objects. More specifically, when lesions are located in the anterior temporal lobe there are greater deficits in the naming of faces and animals. While lesions in left temporoparietooccipital junction condition the appearance of deficits in the recognition and naming of man-made objects such as tools, vehicles and buildings.11
During the surgical procedure of patients with drug-resistant epilepsy these areas are resected, which is why the disorders of the denomination are the most common adverse effects of ATL. Therefore, one of the objectives of neuropsychological assessment in epilepsy surgery is to preserve this language function after surgery.
Functional mapping through EEC is one of the methods aimed at identifying areas associated with language function that may be at risk of being resected in the surgical procedure. The tasks of naming visual confrontation are the most used in the identification of these areas since when stimulated they produce alterations in the naming, indicating cognitively functional regions that must be preserved during the surgical intervention.
We consider that the reports to date underestimate the prevalence of alterations in the denomination due to the failure to use the appropriate tests for valuation. Current studies suggest the presence of selective deficit in the designation of stimuli belonging to different semantic categories. However, to date there is no clarity regarding the categories affected in patients with TLE before and after surgery. This is due to the lack of methods that allow to explore specific semantic subcategories that go beyond the described domains and that they are tested with a suitable population. Here we present a case that exemplifies this problem.
Clinical caseA 26-year-old woman, eutocic delivery, with normal psychomotor development, unreported APGAR score, right hand handedness and undergraduate degree. As a family history of negative for epilepsy and other neurological diseases. She had no relevant pathological personal history. The patient began having seizures at age 19 (2011) with imminent danger sensation, jaw contraction and finger flexors, often once every five months. One year after this (2012), the frequency increased to once a week. At 10 months, the seizures were nocturnal, probably with generalization and the same frequency. In 2014, simple partial seizures characterized by olfactory and visual hallucinations (shadows and macropsies), imminent danger and behavioral arrest.
She was treated with the following antiepileptic drugs in monotherapy and polytherapy in adequate doses, well tolerated, with good adherence but without improvement in the control of the seizures: carbamazepine, oxcarbazepine and topiramate. The last scheme was levetiracetam 2g every 12h and lacosamide 200mg every 12h.
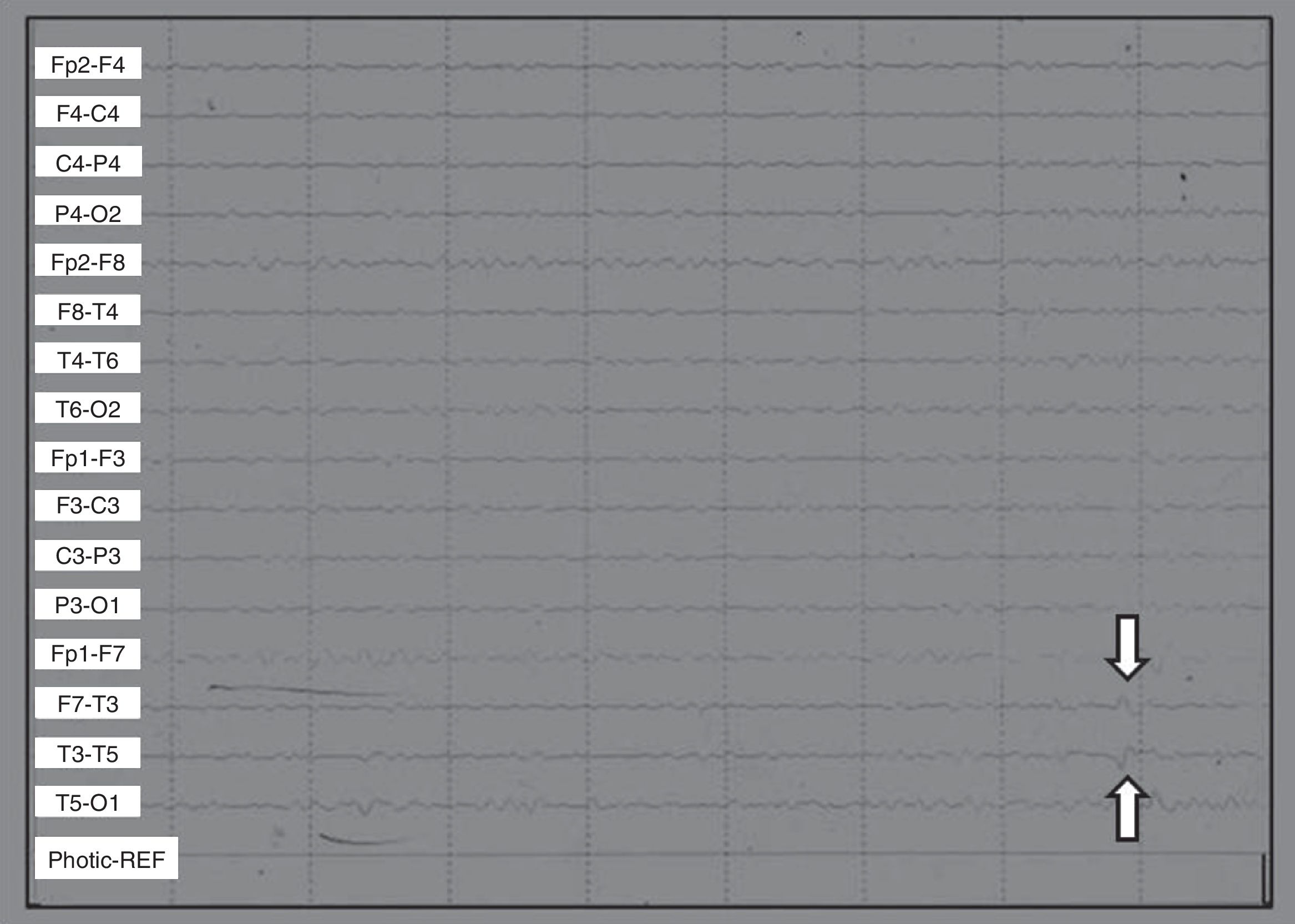
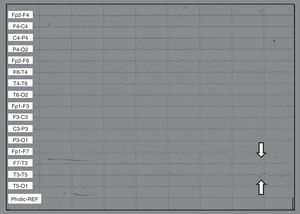
Scalp EEGs showed alpha 10/s activity with paroxysms of low voltage acute waves in the left temporal region. Without changes in hyperventilation, left frontotemporal spikes were triggered by photostimulation (Fig. 1).
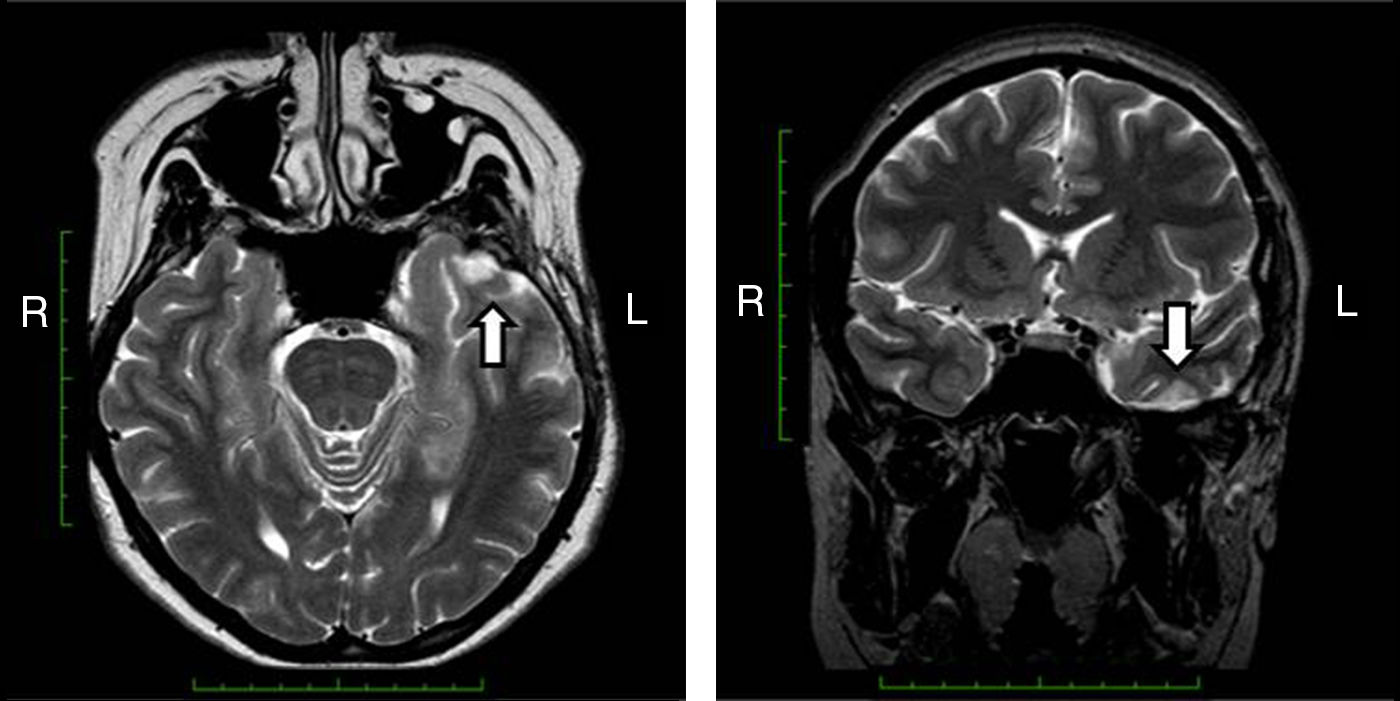
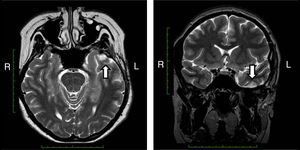
Magnetic resonance imaging (MRI) that included T1-weighted images in the projections, axial T2 and coronal, axial Flair showed a cortical cavernous angioma of the left inferior temporal gyrus in the dorsal region, with areas of surrounding gliosis. The remainder of the study without alterations (Fig. 2).
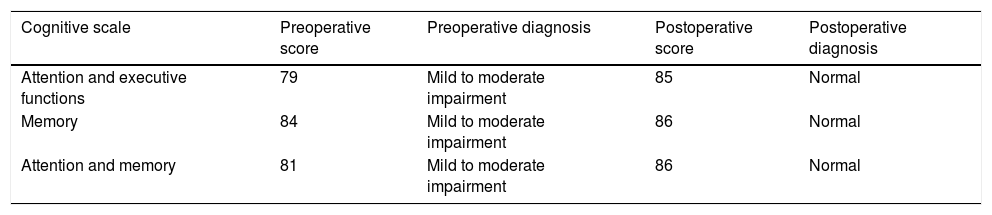
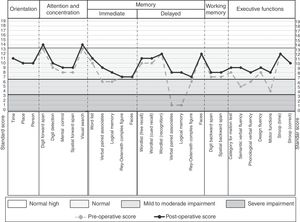
The neuropsychological assessment was performed through the Neuropsi Attention and Memory test before (pre-surgery) and 6 months after surgery (post-surgery). In the 3 scales that make up the test (attention/executive functions, memory and attention/memory) the patient showed pre-surgical scores with mild to moderate impairment. While post-surgical normalized scores were observed on the 3 scales (Table 1).
In the preoperative condition presented mild to moderate impairment in the 3 cognitive domains in comparison to the post-surgical condition in which it shows improvement when obtaining a normal performance in the 3 cognitive scales of the test.
| Cognitive scale | Preoperative score | Preoperative diagnosis | Postoperative score | Postoperative diagnosis |
|---|---|---|---|---|
| Attention and executive functions | 79 | Mild to moderate impairment | 85 | Normal |
| Memory | 84 | Mild to moderate impairment | 86 | Normal |
| Attention and memory | 81 | Mild to moderate impairment | 86 | Normal |
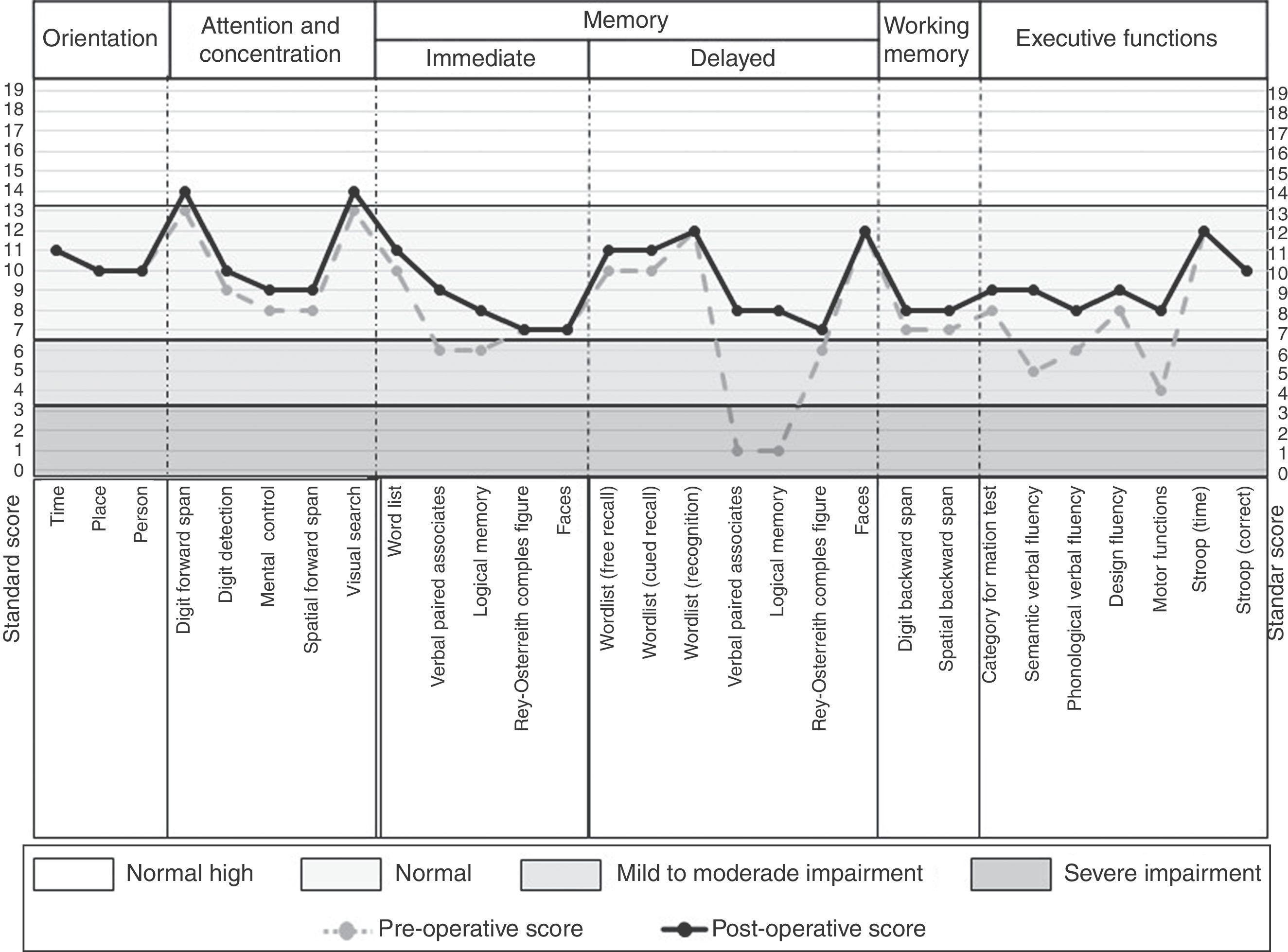
Regarding the neuropsychological profile, in the preoperative condition the patient presented difficulties in verbal memory, planning and organization, cognitive flexibility, working memory, verbal fluency, self-monitoring and metacognition. As well as difficulties in visual confrontation naming. A left frontotemporal neuropsychological deficit was concluded. While at 6 months of surgery it shows a general improvement, obtaining a normal cognitive performance in all the cognitive tasks (Fig. 3).
According to the dichotic listening test, a left hemispheric dominance for language was evidenced.
The case was discussed jointly and interdisciplinarily by neurologists, epileptologists, neurosurgeons, neuroradiologists and neuropsychologists of the Epilepsy Clinic of the General Hospital of Mexico. It was considered to do a cortical mapping of the language through a naming task due to the left hemispheric dominance for the language, the difficulties observed in the neuropsychological assessment (naming deficits) and considering the diagnosis of TLE secondary to cavernoma of the left temporal pole.
Naming taskThis task is composed of 62 images in color and high resolution of real objects. The stimuli are divided into two domains: LT and NLT. The LT domain consists of subcategories of animate SV (animals, insects), inanimate LT (flowers, vegetables, fruits) and body parts. The domain of NLT is composed of the subcategories of clothing, tools and environment (furnitures, vehicles, musical instruments, buildings and kitchen utensils).12
In addition, a pilot test of face names with internal validity was made, consisting of 31 stimuli corresponding to the faces of people belonging to politics, sport and entertainment.
Prior to ES, a practice and baseline stage was performed, randomly presenting the 62 images corresponding to the domains (LT and NLT) and the 31 faces.
The patient should say the name of the image presented to him, for which the examiner wrote down one of three options:
- (a)
Say the name of what you observe. If the initial response was incorrect or vague (e.g. animal, fruit, or flower), he was asked to state more precisely the name of the stimulus (armadillo, strawberry, or tulip).
- (b)
Know the object, but not remember at that time his name. To make sure that there was full recognition, he was asked to describe it. For example, say on a scale of 0–100% if it was familiar or unfamiliar, what it is used for, where it is, how it is, where it has been seen before, and so on.
- (c)
Tip of the tongue, knows the object, knows its name but cannot say (manifest having it on the tip of the tongue).
Based on this, we selected the stimuli that he called and therefore recognized correctly, eliminating those in which he had difficulty naming or recognizing. The correctly named stimuli were used as baseline (Preoperative) and presented to the patient during ES and 6 months after the TLA (Postoperative).
Electrical cortical stimulationThe mapping was performed through the placement of intracreaneal recording meshes in temporal and lateral frontal and temporal basal regions, by means of the Grass stimulator model S88 coupled to Gould 500 Oscilloscope.
Stimulation was applied with Lily square pulses of 1ms width with 60Hz frequency in both meshes.
During the application of the stimulation the images to be named were presented on a 20-inch monitor of a computer through e-Prime 2 software. The presentation time of each stimulus was 4s after a blank screen with a dot fixing. When the patient gave the name of the object was recorded by keyboard as she expressed (whether correct or incorrect). There was no limit to the answer.
The naming percentage was calculated by dividing the number of correctly named stimuli by the total stimulus per 100. The performance of the patient in the naming task was compared before (Pre), during (ES) and 6 months after ES and LTA (Post) through the chi-square test for a sample.
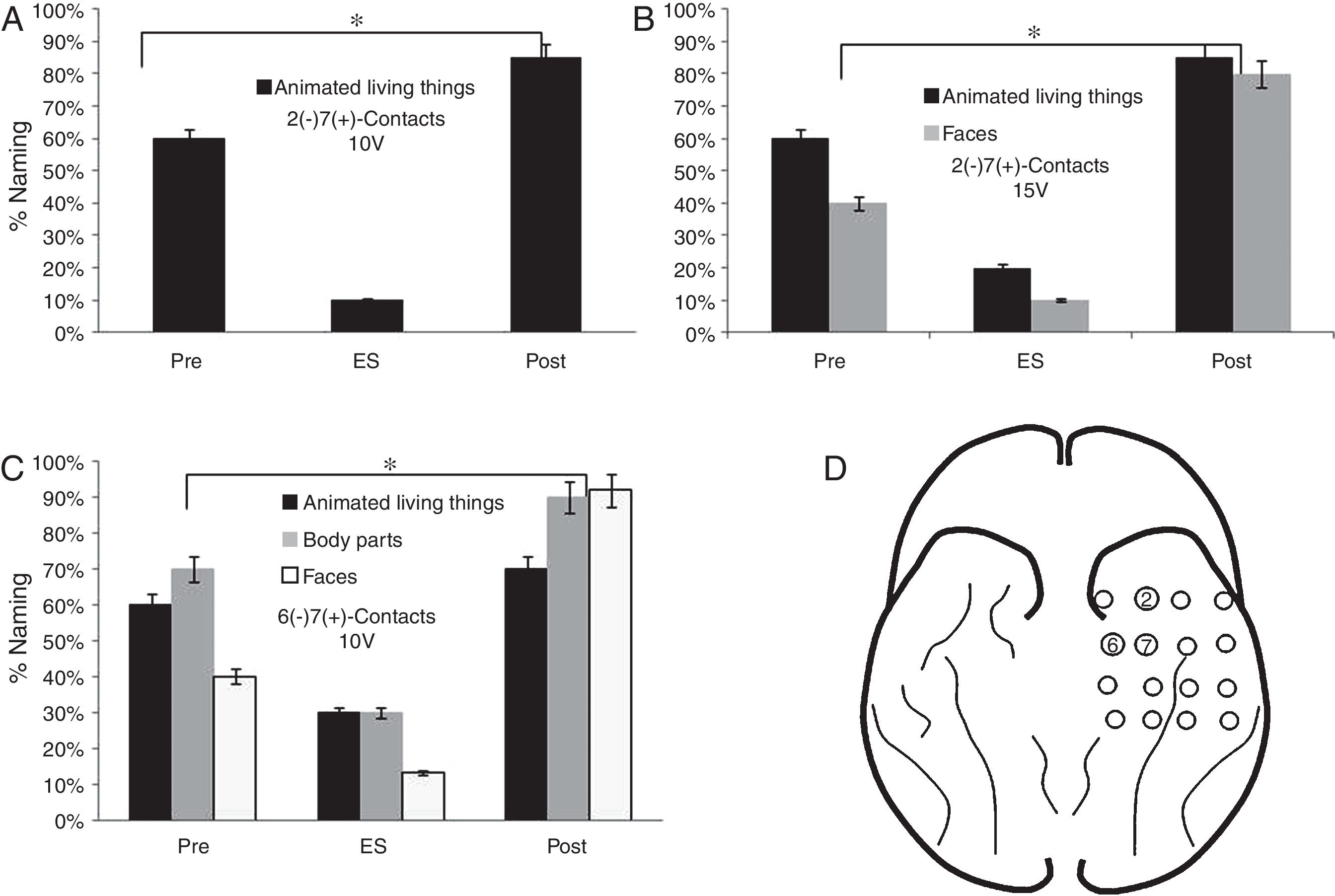
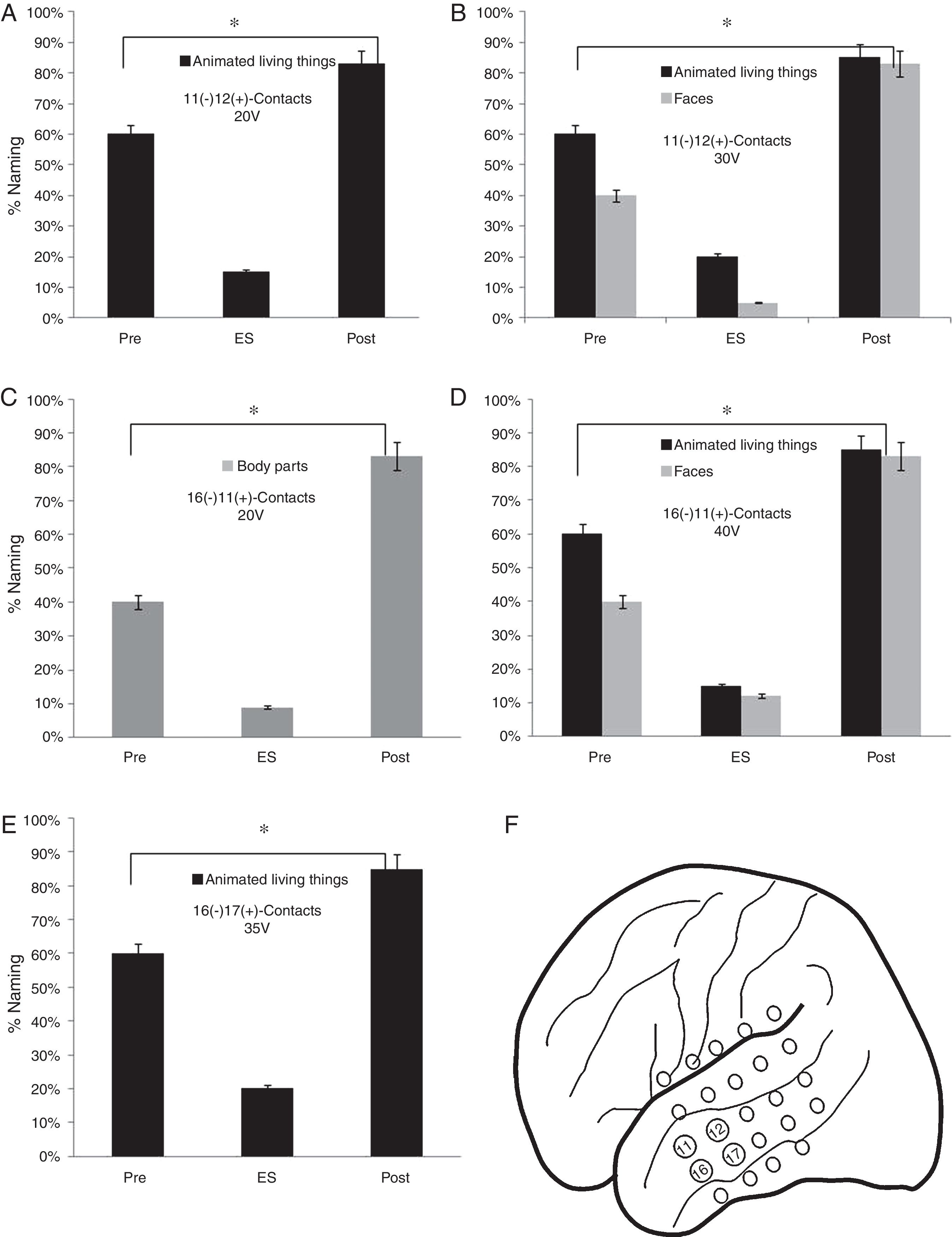
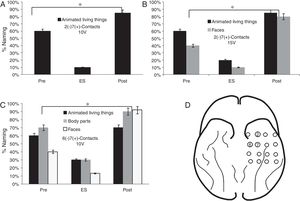
FindingsRegarding the basal mesh, the patient had a lower percentage of face denomination (10%) during the stimulation of contacts 2 (−) 7 (+) from 10V (p<0.05). In the subcategory of LT, there was a lower performance in the denomination of animated LT (20%) and faces (10%) from 15V (p<0.05). When stimulating 6 (−) 7 (+) contacts from 10V, a lower percentage of denomination of faces (13%), body parts and animated LT, respectively (p<0.05) were observed (Fig. 4).
Performance in the naming task in the stimulation of the contacts of the basal mesh: (A) There is a lower performance for the animated LT during stimulation compared to baseline (pre) and 6 months after surgery (p<0.05). (B) It is observed that before the increase of the voltage adds difficulties in the naming of faces (p<0.05). (C) The patient shows increased naming difficulties in animated LT, parts of the body and faces (p<0.05). (D) Contacts that showed difficulties in the naming of animated LT, faces and body parts.
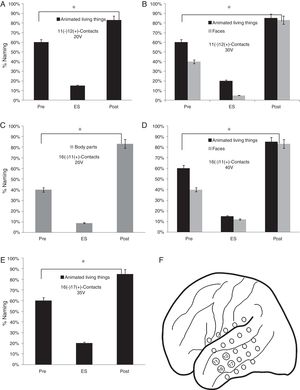
As for the lateral mesh, the stimulation of 11 (−) 12 (+) contacts from 20V showed a lower percentage of face names (15%) (p<0.05). When the stimulation voltage was increased to 30V, the patient presented a worse performance in the naming of faces (5%) and animated LT (20%) (p<0.05). A worse performance was found in faces naming (9%) by stimulating the contacts 16 (−) 11 (+) from 30V and faces (12%) and animate LT (15%) from 40V (p<0.05). Finally, a lower naming percentage was observed by stimulating 16 (−) 17 (+) contacts from 35V in the animated LT category (20%) (p<0.05) (Fig. 5).
Execution in the naming task in the stimulation of the contacts of the lateral mesh: (A) There is a lower performance in the naming of the animated LT (p<0.05). (B) When the voltage increases, difficulties appear in the denomination of faces (p<0.05). (C) The patient shows deficits in the naming of body parts without showing problems in other categories (p<0.05). (D) The increase in voltage causes greater difficulties in the naming of animate LT and faces but without alterations in the naming of body parts (p<0.05). (E) Decrease in the naming percentage of the animated LT domain with no affection in other categories (p<0.05). (F) Contacts in which the patient presented naming deficits on faces, animated LT and body parts.
The naming percentage for the SNV domain was higher than 85% in its three categories (tools, clothing and environment) before, during and after ES.
Facial and tongue motor response was found by stimulating 10 (−) (5+) contacts.
In this way it was concluded that the patient could be a candidate for resection of the lesion. The recording meshes were removed and the left temporal cavernoma was resected. In the postoperative period, it was oriented in time space and person, isometric pupils, with present photomotor and consensual reflex, facial symmetry, mobilization of the four limbs and without data of neurological complications. He did not present neuropsychological deficits (memory and language) after the surgery (6 months later) objectified through the Neuropsi Attention and Memory test and the task of naming specific categories.
DiscussionThe patient showed greater difficulties in naming faces, animated LT and body parts during ES compared to baseline and six months after surgery. This suggests that the epileptic activity produces affection in the recovery of the words, attributing this function to the left temporal pole.
The voltage increase in the contacts: 2 (−) 7 (+) 15V, 11 (−) 12 (+) 30V and 16 (−) 11(+) 40V, produced deficits in animated LT and faces but not in other categories. This seems to indicate that changes in voltage can selectively affect naming and that certain categories are more sensitive to variations of this parameter.
Alterations in naming in patients with TLE are selective by affecting different semantic categories and the conservation of others (NLT and inanimate LT). Therefore, such problems can be used as indicators of neuropsychological deficits in TLE, thus suggesting the lateralization and circumscribed area of the epileptic focus.
Difficulties in naming after surgery have been detected a few weeks later, six months and one year after the procedure.13
Studies carried out by Laws (2003) have shown naming impairment of stimuli belonging to the category of fruits and vegetables but not of animals.14 Other investigations have found dissociations between small and manipulative objects like tools and large and non-manipulable objects like buildings. As well as selective deficits between objects typical of the interior of a house and objects of the environment.15
On the other hand, the most used neuropsychological test and considered the gold standard for the naming assessment is the “Boston Naming Test” (BNT). However, this test is not specific since it contains mostly stimuli corresponding to the NLT domain, which are inadequate to examine the brain regions involved in naming, which are the most pressing and interesting in LTE surgery.
The findings of the present report have demonstrated the existence of a clear and notorious differentiation of the organization and processing of semantic knowledge, reflecting a more detailed neurocognitive network than the simple living/non-living dichotomy. This allows identifying categories affected by epileptic activity and its anatomic-functional association to certain regions of the brain (particularly left temporal pole), areas that should be considered during surgical resection in the LTA.16
This study is important given the limited number of reported work by category and CEE. Until recently it was only considered whether or not the patient was named during ES. With the studies of specific categories we know that this is not enough since the damage is selective, thus enabling the characterization of preoperative neuropsychological diagnosis in patients with TLE (localization and indirect lateralization of cognitive affection and preserved functions).
ConclusionsThe combination of neurocognitive techniques such as the tasks of category naming together with the ES allows to delimit specific areas to be considered in the surgical plan, evidencing regions that participate in the recovery of the words and thus to estimate the effect of the surgery in the naming (areas cognitively functional in relation to the location of the epileptic focus to be resected).
Consisting of the neurocognitive findings, it was possible to plan the resection of the lesion without postoperative cognitive deficits. This result could not have been realized if the surgical team did not have this information obtained from the brain mapping. Therefore naming is one of the aspects that should be taken into account in the neuropsychological assessment in TLE.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestsNone of the authors has any conflict of interest to disclose.
Our sincere thanks to the neuropsychology team for the support provided in the technical support