Gastrointestinal stromal tumors (GISTs) are rare mesenchymal tumors of the alimentary tract. Nowadays GISTs represents 0.1–3% of all gastrointestinal malignancies, making it a diagnostic challenge. Lesions are frequently located in stomach and proximal small intestine but rarely elsewhere in the abdomen. They are believed to result from mutations of proto-oncogenes c-Kit or platelet-derived growth factor receptor alpha polypeptide, this increase tyrosine kinase receptor activity, leading to uncontrolled proliferation of stem cells that differentiate into cells of Cajal. They can occur at any age but predominantly in middle-aged people and in elderly. We report the case of a 28-year-old male presented to our hospital with upper gastrointestinal bleeding, findings in diagnostic image studies suggested a gastric GIST without evidence of metastatic disease; therefore totally tumor excision was performed. Cytologic and inmunohistochemistry analysis confirm diagnosis of GISTs.
Los tumores del estroma gastrointestinal (GISTs) son neoplasias raras de origen mesenquimal en el tracto alimentario. Típicamente se localizan en estómago e intestino delgado proximal, con pocos casos en otros sitios en abdomen. Se sabe que los GIST pueden ser el resultado de mutaciones de proto-oncogenes c-Kit o del receptor alfa polipeptídico del factor de crecimiento derivado de plaquetas, esto aumenta la actividad de receptor tirosincinasa y lleva a una proliferación descontrolada de células madre que se derivarían a Células de Cajal. Ocurren a cualquier edad pero son frecuentes en la mediana edad y en la vejez. Reportamos el caso de un paciente masculino de 28 años de edad quien inicia con hemorragia de tubo digestivo alto, los hallazgos en los estudios de imagen sugirieron un GIST gástrico sin evidencia de enfermedad metastásica, se realizó excisión total del tumor. Análisis citológico e inmunohistoquímico positivos para GIST.
Stromal or mesenchymal tumors that affect the gastrointestinal tract typically appear as subepithelial neoplasms and they are classified in two groups. The most common is Gastrointestinal Stromal Tumors Group (GIST), which arise from mesenchymal stem cells programmed to differentiate into interstitial cells of Cajal in the myenteric plexus.1 The cells of Cajal form a complex cell network within the gastrointestinal tract wall where they function as a pacemaker system. The GIST can arise from anywhere into the gastrointestinal tract and are frequently located in stomach (66%) and small intestine (30%) particularly in duodenum, as well as in esophagus, colon, anus and rectum (<10%).2 They can even occur out of the digestive tube, by example in omentum, mesentery or peritoneum.3–6
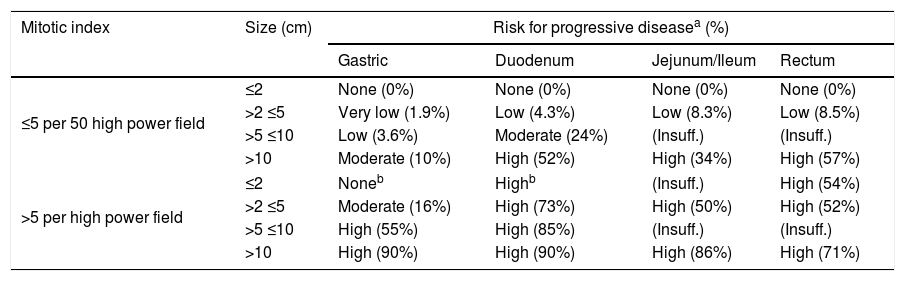
GIST are characterized by exhibit proto-oncogene proteins c-Kit mutations (CD117 and/or CD34), and also platelet-derived growth factor (PDGF-alpha). CD117 detected by immunohistochemistry confirms the diagnosis of GIST.2 The tumor size by itself, does not predict the GIST biological behavior. However, the mitotic index (Table 1), the size and location of tumor are used together with the end to predict the biological behavior and the risk of disease progression.7,8,22,26
Risk stratification of primary GIST by mitotic index, size and site [Miettien and Lasota, 2009].
| Mitotic index | Size (cm) | Risk for progressive diseasea (%) | |||
|---|---|---|---|---|---|
| Gastric | Duodenum | Jejunum/Ileum | Rectum | ||
| ≤5 per 50 high power field | ≤2 | None (0%) | None (0%) | None (0%) | None (0%) |
| >2 ≤5 | Very low (1.9%) | Low (4.3%) | Low (8.3%) | Low (8.5%) | |
| >5 ≤10 | Low (3.6%) | Moderate (24%) | (Insuff.) | (Insuff.) | |
| >10 | Moderate (10%) | High (52%) | High (34%) | High (57%) | |
| >5 per high power field | ≤2 | Noneb | Highb | (Insuff.) | High (54%) |
| >2 ≤5 | Moderate (16%) | High (73%) | High (50%) | High (52%) | |
| >5 ≤10 | High (55%) | High (85%) | (Insuff.) | (Insuff.) | |
| >10 | High (90%) | High (90%) | High (86%) | High (71%) | |
Notes: Insuff.: Insufficient data.
In this study, we report the case of a uncommon age presentation of this tumor.
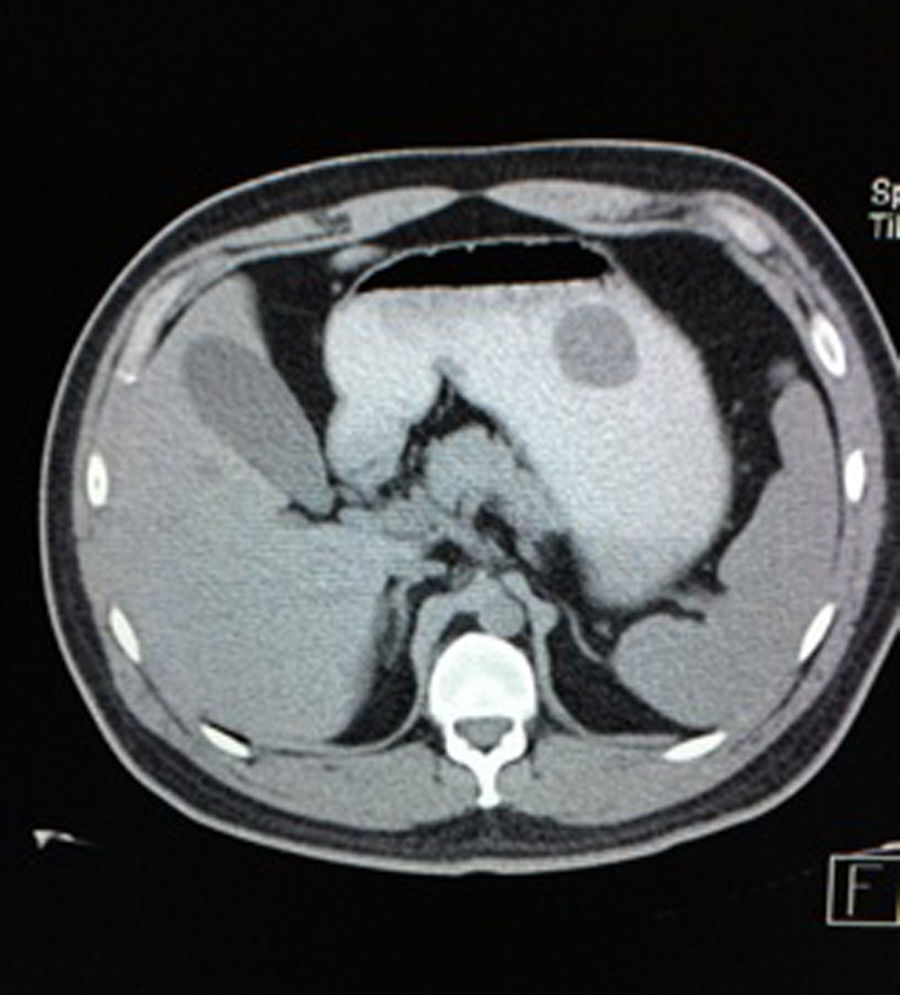
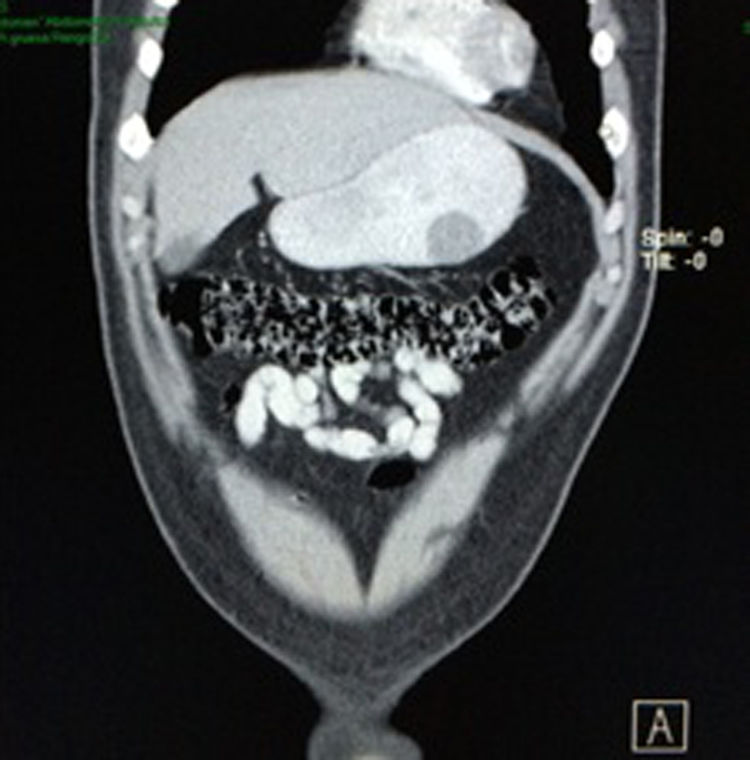
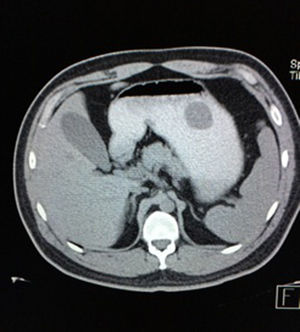
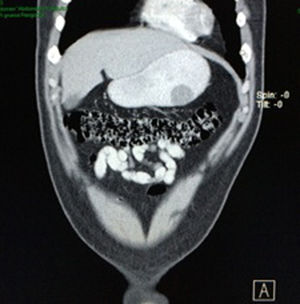
Case reportA 28-year-old male patient, presented to the Emergency Room at the Hospital General de México “Dr. Eduardo Liceaga”, with 1-day history of melena (three episodes in 24h), no nausea, no vomiting, no hematemesis, neither fever. His medical history was unremarkable and he has not recently used non-steroidal anti-inflammatory or steroid drugs. He does not have family history of note and was not on regular medication. Current or previous use of recreational drug and history of chronic liver disease or peptic ulceration were denied. Venous blood tests revealed a hemoglobin level of 8g/dL then one unit of packed red blood cells was transfused. After resuscitation and stabilization, he underwent to upper GI endoscopy under sedation. A bleeding gastric mass with irregular borders was identified along the greater curvature of the stomach. Later, the patient was moved to General Surgery Service, following this, he was brought under to abdominal contrast enhanced CT Scan. The images showed a homogeneous pattern attenuation range of soft tissue (35uH), and a well-defined dumb-bell shaped 3.7×3.1cm mass arising from the greater curvature of stomach and well attached at it. There was no evidence of distant lesions (see Figs. 1 and 2).
Afterwards blood tests revealed WBC of 6.6×10E3/μL, Hb 9.5, HCT 29.2%, platelet count of 322 109L–1, PT 12.1s, aPTT 23.8s, 89.7% activity, INR 1, ALT (SGPT) 44U/L, AST (SGOT) 29U/L, AP 92U/L, GGT 27U/L, LDH 145U/L, Na 126mEq/L, K 3.5mEq/L, Cl 104mEq/L, glucose 81g/dL, urea 23.5mg/dL, creatinine 1.1mg/dL, direct bilirrubin 0.1mg/dL, indirect bilirrubin 1.09mg/dL, total bilirrubin 1.19mg/dL, albumin 4.2g/dL.
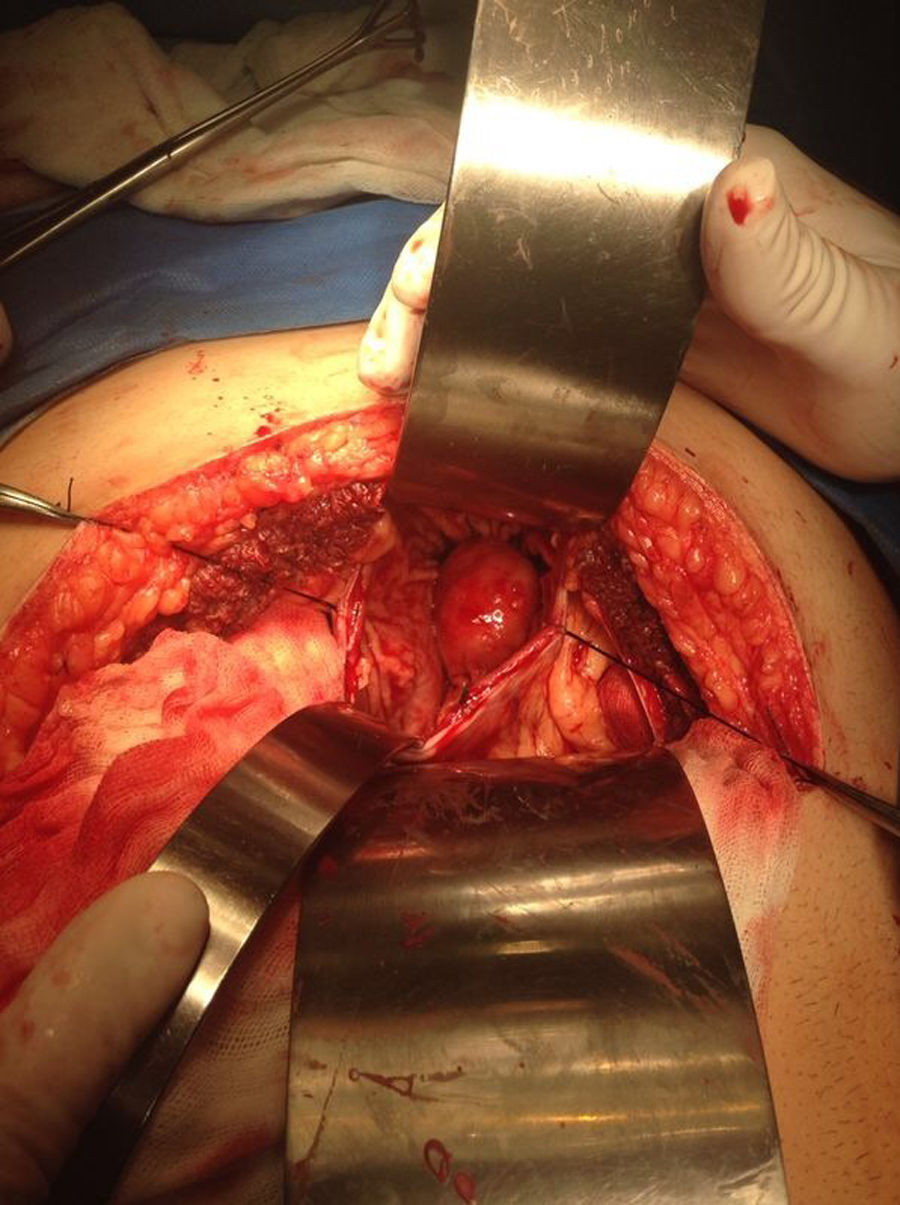
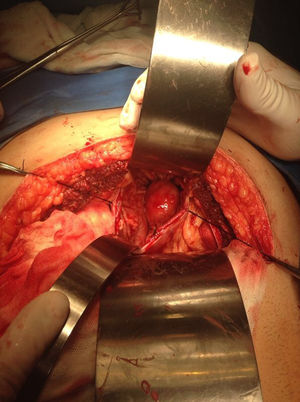
Our surgery plan was to perform a transgastric tumorectomy by using a Chevron type approach (see Fig. 3). Adhered to the posterior gastric wall at the greater curvature, a well-defined edges and soft consistency 4×3cm tumor was found (see Fig. 4). There was no evidence of peritoneal or liver metastases. After totally tumor excision that included a proximal gastrectomy, which it was closed in two planes with absorbable multifilament suture, a closed active drainage (i.e. Jackson-Pratt) facing epigastric and externalized toward left flank was placed. Closing fascia with 1–0 PDS continuous surjete and skin with 3–0 nylon Sarnoff points.
Postoperatively, the patient had an uneventful recovery with no evidence of leak from his anastomosis. Then, on the third day, he started a liquid diet and subsequently a soft diet on the fourth day. The drain was removed on the sixth postoperative day. Finally, considering the satisfactory recovery, he was discharged seven days after surgery without complications.
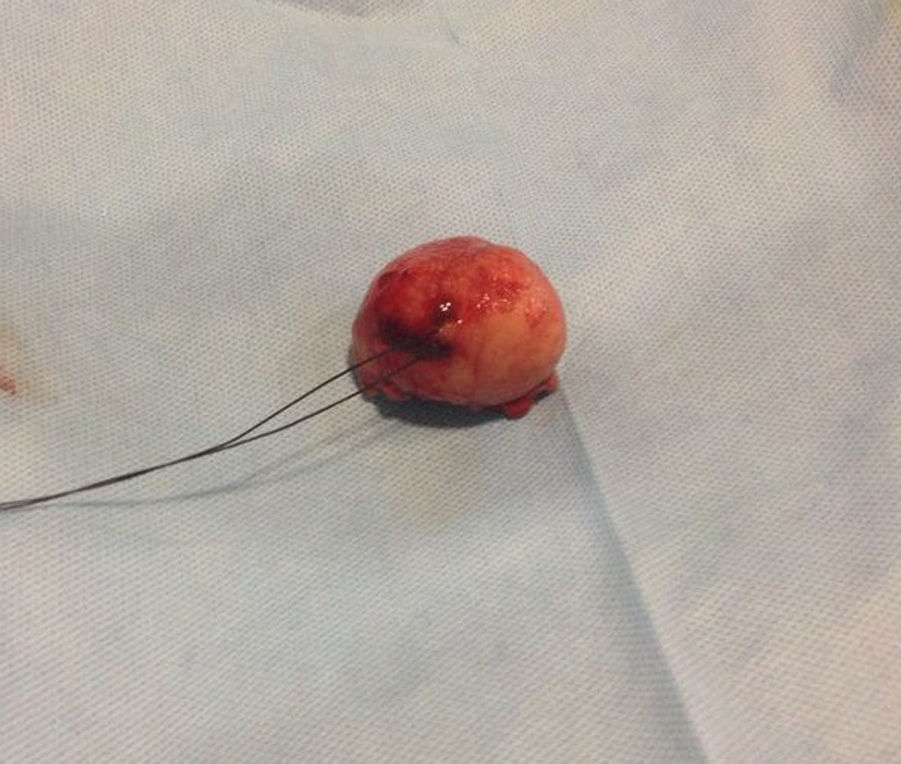
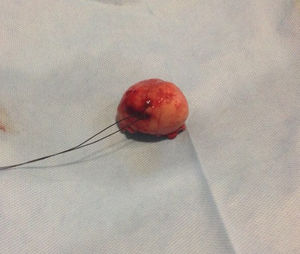
Pathologic analysis of the removed neoplasm measured 4.5cm on the major axis. Histopathology revealed that the tumor is compatible with Gastrointestinal Stromal Tumor (GIST). The mitotic index was 2 in per 50 high-power-field, the tumors margins were clear, the lymphovascular gastritis not related to H. pylori was found and there was no evidence about lymphovascular invasion.
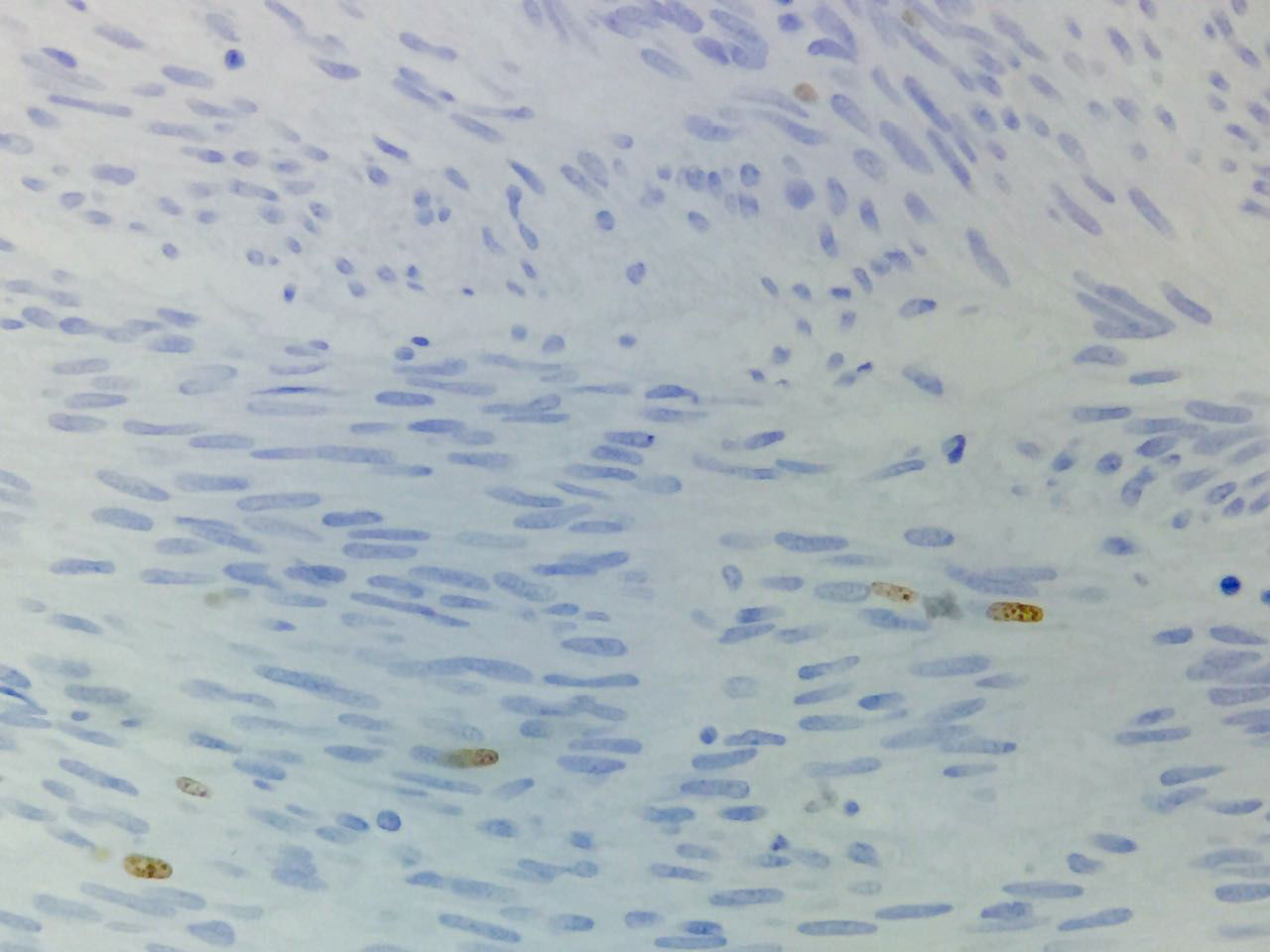
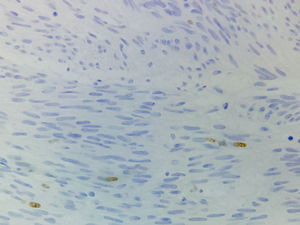
Immunohistochemical studies revealed tumor neoplastic cells stained positive for KIT (CD117) and Ki-67 at 2% (see Fig. 5), while CD34, smooth muscle actin and S-100 protein were negative.
DiscussionGastrointestinal stromal tumors are neoplasms with a genetic origin. They are very similar to other family oncologic syndromes like NEM 1 and 2, VHL, and Carney complex (e.g. gastric leiomyosarcoma, pulmonary, and functioning extra adrenal paraganglioma). Consequently, GIST pathophysiology follows a common pathway with other mesenchymal neoplasms of the gastrointestinal tract and so, this may lead to obscure the diagnosis or to being confused with leiomyoma or leiomyosarcoma. Immunohistochemical resources (i.e. KIT, CD117, CD34, S-100 protein, Actin, Desmin, etc.), and imaging studies are crucial for getting a right diagnosis.1
Despite that the GIST are the most common benign not epithelial neoplasm of the gastrointestinal tract, it just represents 1% of all gastrointestinal primary tumors.2 With a worldwide incidence of 11–19.6 per million populations, we do not know incidence and prevalence in Mexico. However, in USA, recent studies estimate an annual incidence of 4000 to 6000 new cases (7–20/1,000,000 per year). Typically, GIST affects the population over 50-years old, rarely those under 40s, and the average age of diagnosis is around 63 year-old.7,9–11
The case under study corresponds to a male patient in his third decade of life with a gastric GIST in a habitual localization, notwithstanding the unusual age of the patient. Clinically, this type of GIST usually begin with symptoms like upper gastrointestinal bleeding, followed by abdominal chronic pain, and when the tumor size is considerably big it could be detected during abdominal manual exploration.12,18–20
GIST are characterized by staining positive by KIT and some of them by PDGFR-alpha. In 1998, Hirota et al. showed the existence of mutations in these kind of tumors, by proving that the KIT mutation stains positive on 60–80% of GISTs cases.2,12,32,33 In normal cells, the activity of KIT tyrosine kinase receptor is regulated by ligand dependent activation. On the other hand, on GIST a gain of function mutation in the exon 11 in the juxtamembrane domain of the c-Kit gene leads to the consecutive ligand-independent activation of KIT receptor kinase, which may cause tumorigenesis.2,14,15 Imatinib mesylate is a potent and selective inhibitor of tyrosine kinase (including KIT, BCR-AL, and PDGFR-alpha).16,17 By considering that GIST are not sensitive to radio or chemotherapy, the patients in whom the surgical resection was not enough have increased their survival rate since Imatinib FDA approval.2 Staining positive for CD117 confirms diagnosis of GIST. The absence of intense and diffuse activity for S-100 rejects melanoma, which is important because this entity commonly promotes metastatic activity in gastrointestinal tract. Tumor size by itself does not predict its biologic behavior2; it is well known that even <5cm tumors can generate metastatic activity. Nevertheless, the mitotic index, tumor size and site are commonly used together with the aim to predict the biologic behavior of tumor and the disease progression risk too1,8,21,22,26 (Table 1).
Nowadays, we have imaging diagnosis methods as endoscopy, endoscopic ultrasound, computed tomography scan, and magnetic resonance tomography. These methods have become crucial for the diagnosis of subepithelial neoplasms, such as the case of GIST.23–25,27,28 When the imaging studies reveal suggestive features of GIST, and the tumor is considered resectable by size, localization or any other parameter, biopsy should not be performed due to the imminent risk of rupture and intraabdominal spread. Tumor size >4cm, irregular borders, echogenicity foci and presence of cysts are suggestive features of malignancy.29–31
In absence of metastatic disease, the GIST curative treatment is the complete surgical resection.2,5,13 The first symptom of patient was the upper gastrointestinal bleeding. Then, the tumor was revealed by endoscopy and CT scan. Moreover, the metastatic activity was discarded and in accordance with the imaging study, the biopsy was not required. Histopathologic features were compatible with GIST and immunohistochemical analysis of the tumor stained positive for KIT (DC117) in an intense way. Furthermore, the tumor stained positive for Ki-67 at 2%, this molecule is a nuclear protein present in all active phases of cell cycle (G1, S, G2 and mitosis), but is absent in cells on latent phase and undetectable during the DNA repairing process. The activity percentage of Ki-67 point out the presence of tumor proliferation (activity ≥15% is related to malignancy).33,34
Due to the physiopathologic and immunohistochemical tumor features, the patient was diagnosed with gastric GIST, in accordance with the National Comprehensive Cancer Network. Surgical resection was the best treatment option for him and could be considered curative for this particular case, by having a very low risk for progressive disease (i.e. 1.9%). The patient remains well after six months following surgery. Imatinib mesylate was not indicated as neoadjuvant treatment in the case under study. This work reveals the necessity of early recognition and appropriate investigation of gastrointestinal symptoms at all age groups for excluding potential malignant causes.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis work did not receive any kind of financial support from sources like grants from foundations, scholarships, and others.
Conflict of interestWe do not have any financial or personal relationship which can cause a conflict of interest regarding this article.
We would like to thank Dr. Nicol Simone-Renzi and Dr. Noe Israel Cano-Zepeda for their contribution to this paper.