The resurgence of tuberculosis and the appearance of multidrug-resistant strains of Mycobacterium tuberculosis are due to several different factors. Principal among these is the worldwide prevalence of autoimmune deficiency syndrome (AIDS), coupled with misdirected therapeutic practices and patient non-compliance, the latter being derived from the undesirable side effects of anti-tuberculosis drugs. Aside from AIDS, other diseases or immunosuppressive disorders have been associated with this new phase of tuberculosis infection. Metabolic disorders such as diabetes, which is closely associated with obesity, are among the most important immunosuppressive diseases. Diabetes is a major public health problem in Mexico, a country that leads the world in the incidence of childhood obesity.
The aim of this study is to analyse the different factors involved in this phenomenon and to give a clear, comprehensive picture of the problem of tuberculosis resurgence and its correlation with diabetes and metabolic syndrome.
El resurgimiento de la tuberculosis y la aparición de nuevas cepas de Mycobacterias poliresistentes tienen un origen multifactorial. Entre las principales causas involucradas se encuentran el alto número de casos de pacientes con Síndrome de Inmunodeficiencia Adquirida (SIDA) a nivel mundial, así como incorrectas prácticas terapeúticas y la falta de apego a ellas por parte del paciente, esta última derivada de los indeseables efectos secundarios de los antituberculosos. Independientemente de los casos de SIDA, otras enfermedades o condiciones inmunosupresoras han sido de igual forma relacionadas con esta nueva fase de la tuberculosis. Dentro de las principales enfermedades que propician un estado de inmunodepresión en el paciente se encuentran las enfermedades metabólicas como la diabetes, esta condición está estrechamente relacionada con la obesidad, problema de salud pública del cual en su forma infantil México ocupa el primer lugar a nivel mundial. La presente investigación documental tiene como objetivo aportar elementos de análisis que nos acerquen a un entendimiento integral de la problemática del resurgimiento de la tuberculosis y su correlación con diabetes y síndrome metabólico.
Metabolic syndrome is a cluster of risk factors that include hyperglycaemia/insulin resistance, high blood triglycerides, low levels of HDL cholesterol, abdominal or visceral obesity, and arterial hypertension.1,2 Diagnosis of metabolic syndrome is important, as it can identify patients at high risk for cardiovascular disease and type 2 diabetes mellitus (DM).2 Approximately 1 in every 4–6 adults develops metabolic syndrome, and it affects around 30% of individuals over 50 years in Europe, and 40% in the USA.3,4
Tuberculosis (TB) is the most devastating infectious disease known to man, and was the primary cause of death in Europe in the 19th century.5 Incidence of this disease is currently on the rise due to an increase in the number of patients with immune deficiency secondary to human immunodeficiency virus infection (HIV),6 which in turn increases the risk of a resurgence of Mycobacterium tuberculosis infection.6
In 2008, 14,986 new cases of tuberculosis were reported in Mexico, with 4896 deaths from the disease.7 The relationship between diabetes and tuberculosis has been known for decades; a study published by Root as far back as 1934 found that nearly 85% of the 245 study patients with both diseases had developed tuberculosis after being diagnosed with diabetes mellitus.7
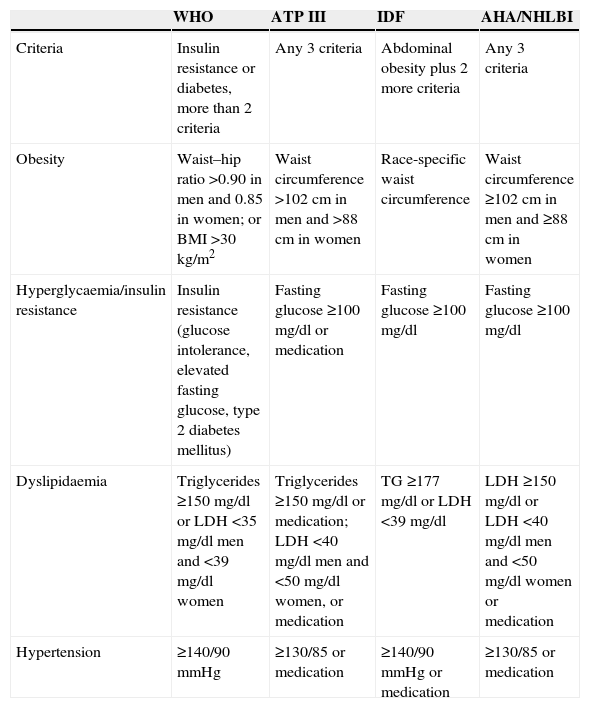
Metabolic syndrome and diabetes mellitusThe World Health Organisation (WHO), the National Cholesterol Education Programme Adult Treatment Panel III (ATP III), the International Diabetes Federation (IDF), and the American Heart Association/National Heart, Lung and Blood Institute (AHA/NHLBI) have defined a number of parameters associated with metabolic syndrome (Table 1).
Comparison of diagnostic criteria for metabolic syndrome.
| WHO | ATP III | IDF | AHA/NHLBI | |
|---|---|---|---|---|
| Criteria | Insulin resistance or diabetes, more than 2 criteria | Any 3 criteria | Abdominal obesity plus 2 more criteria | Any 3 criteria |
| Obesity | Waist–hip ratio >0.90 in men and 0.85 in women; or BMI >30kg/m2 | Waist circumference >102cm in men and >88cm in women | Race-specific waist circumference | Waist circumference ≥102cm in men and ≥88cm in women |
| Hyperglycaemia/insulin resistance | Insulin resistance (glucose intolerance, elevated fasting glucose, type 2 diabetes mellitus) | Fasting glucose ≥100mg/dl or medication | Fasting glucose ≥100mg/dl | Fasting glucose ≥100mg/dl |
| Dyslipidaemia | Triglycerides ≥150mg/dl or LDH <35mg/dl men and <39mg/dl women | Triglycerides ≥150mg/dl or medication; LDH <40mg/dl men and <50mg/dl women, or medication | TG ≥177mg/dl or LDH <39mg/dl | LDH ≥150mg/dl or LDH <40mg/dl men and <50mg/dl women or medication |
| Hypertension | ≥140/90mmHg | ≥130/85 or medication | ≥140/90mmHg or medication | ≥130/85 or medication |
According to the WHO, insulin resistance is the primary risk factor and is a pre-requisite for a definitive diagnosis of metabolic syndrome.8,9 For the ATP III, however, diagnosis is confirmed when any 3 of the 5 risk factors are present.9–11 The IDF made abdominal obesity a prerequisite for diagnosis of metabolic syndrome,9,12 while the AHA/NHLBI modified the ATP III criteria and does not require any particular factor as a prerequisite for diagnosis.9,13,14
Elevated fasting glucose, one of the conditions included in metabolic syndrome, was long thought to be the primary predictor of diabetes. However, Lorenzo et al. showed that taking all metabolic syndrome factors together had a superior predictive value for the development of diabetes. Ford, Li and Sattar, meanwhile, concluded that diagnosis of metabolic syndrome was a more accurate predictor of development of diabetes than of cardiovascular disease.15,16 The WHO has described DM as an epidemic that primarily affects developing countries. Diabetes is known to increase the risk of developing infection, possibly as a result of changes in cellular immunity.17
TuberculosisTuberculosis is one of the most prevalent infections worldwide, and was declared a global emergency by the WHO in 1994.18,19 Approximately 2 billion people are infected with the M. tuberculosis bacterium, 8 million new cases are reported each year, and the disease causes around 2 million deaths annually.20
Estimates suggest that between 10% and 30% of TB patients also present DM, caused by an impaired immune response.21,22 In TB patients, this impairment is thought to be caused by reduced macrophage activation, which diminishes the body's capacity to destroy the tubercle bacilli, together with lower levels of tumour necrosis factor alpha and nitric oxide.23
Growing evidence of the association between these diseases has made it imperative to clarify the relationship between metabolic syndrome, DM and TB. This will give insight into the different immune response changes caused by these metabolic diseases and shed light on the altered course of TB. This will enable clinicians to take more a comprehensive therapeutic approach in patients with these concomitant pathologies, and reduce mortality by giving more appropriate medication.
Several studies have suggested that the Th1-type, and to a lesser extent the Th17-type immune response, aided by cytokines such as IL-12, IFN-γ, TNF-α and IL-17 and IL-23, are the best defence against M. tuberculosis infection.24,25 In the case of TB concomitant with DM, the Th1 and Th17 responses are induced as a result of the immunodepressive effect of diabetes, which in turn increases immune pathology in TB infection.26
A proposed model for TB susceptibility in DM is based on a defective innate response in diabetic patients, resulting in a critical delay in priming adaptive immunity. Within the first 3 days following infection, the phagocytic and chemokine expression (particularly CCL5 and CCL2) capacity of alveolar macrophages is severely reduced. This delays macrophage, dendritic cell (DC) and neutrophil recruitment on days 5–7. On days 9–11, DC transport to lymph nodes is delayed, which in turn delays activation of TB-specific T-lymphocytes until day 15 (in a patient with TB alone, this would happen on days 12–14).27
In patients with TB alone, T lymphocytes will have been transported to the lung by day 15. In TB + DM patients, however, this is delayed until day 21. By week 8, an ineffective response to TB infection, characterised by IFNγ, TNFα and IL1β, is observed.27
Tuberculosis concomitant with diabetesDiabetes mellitus is known to be a risk factor for infection; however, its effect on mortality is less clear.28 In the case of community-acquired pneumonia, the study published by Yende et al. showed higher mortality during the first year, due to a process in which onset of acute infection worsens the preexisting cardiovascular diseases typically found in diabetics.28
In the study published by Alisjahbana et al., the authors conclude that patients with type 2 DM are at high risk for developing active tuberculosis.21 As far as clinical manifestations are concerned, patients with type 2 DM present more symptoms than those with TB alone. However, there was no evidence of more severe infection on microbiological culture and imaging tests.21
Similarly, Stevenson et al., in an analysis of data for India collected by the WHO, estimated that diabetes accounts for 14.8% of cases of pulmonary TB in that country. Furthermore, 18.4% of TB patients in India present concomitant diabetes, even though the national prevalence for this disease is just 4.3%. The authors conclude that the growing diabetes epidemic may lead to a resurgence of tuberculosis in endemic regions. This has potentially serious implications for tuberculosis control, and adequate treatment of these diseases must become a priority.29
The findings of the meta-analysis published by Jeon and Murray confirm that risk for TB is up to 3 times higher in DM patients in all regions worldwide. Thus, DM now presents a risk comparable with that of HIV/AIDS, since although the latter increases the risk of TB infection by anything from 2 to 9 times compared with diabetes, according to estimates for 2007, 33 million people worldwide suffer from DM.30
In the same vein, Restrepo et al. found that patients with diabetes are up to 8 times more likely to develop pulmonary TB. The authors attribute the impaired immune response of DM patients to hyperglycaemia, which is characterised by elevated levels of IFN-γ caused by changes in Th1 cytokine regulation resulting from an increase in advanced glycation end products that modify protein function.31
Therefore, although increased cytokine levels would contribute to mycobacterial elimination, an altered immune response and the presence of dysfunctional cytokines prevent the patient's immune system from responding to the challenge. This could explain the different presentation of TB in patients with and without DM.31
In their study, the authors describe how a mouse model for diabetes showed higher bacterial burden and higher IFN-γ levels in the advanced stages of the disease. This could also hold true of patients with diabetes and chronic hyperglycaemia, although they were unable to confirm the hypothesis due to the low sensitivity and specificity of direct smear tests in assessing bacterial load.31
The study by Young et al, in a sub-Saharan population, found an altered immune response in diabetic patients, in addition to low leucocyte and polymorphonuclear neutrophil levels, and even a decreased Th1 cytokine response to TB. The authors go on to say that co-presentation with TB and DM has also been associated with more severe TB symptoms, such as increased lung cavitations and longer periods of smear positivity.32
The most comprehensive analysis of the relationship between immune, endocrine and metabolic alterations in TB was carried out by Santucci et al. In this study, the authors found elevated levels of IFN-γ cytokines, IL-10, IL-6 and C reactive protein, cortisol, and the growth hormone, together with reduced levels of leptin, testosterone and dehydroepiandrosterone (DHEA). Recent studies have determined that DHEA facilitates the Th1 response and has a potent anti-inflammatory effect. Reduced levels of DHEA in TB, therefore, are associated with impaired protection against tissue damage and an impaired immune response.20
Effects of comorbidity on treatmentCo-existence of both diseases has been associated with poorer prognosis and less therapeutic efficacy.18 For this reason, both the WHO and the International Union Against Tuberculosis and Lung Disease have called for international guidelines on the management and control of tuberculosis and diabetes.22
The collaboration between these organisations resulted in the Collaborative Framework for Care and Control of Tuberculosis and Diabetes. This document provides the basic information needed to treat both diseases simultaneously as an interim measure before international treatment guidelines can be drawn up. The Collaborative Framework recommends that patients with DM be monitored for signs of TB, and vice versa. It also recommends the use of directly observed treatment short course (DOTS), which is suggested to improve both the management and treatment of comorbid patients.33
Various studies have described the poor prognosis and therapeutic difficulties associated with these comorbidities, showing the obstacles encountered in the management and treatment of TB combined with DM.
The study conducted by Reis-Santos et al. in a Brazilian population found that treatment failed in 20% of study patients.18 Similarly, the Jiménez-Corona study found the relative risk of treatment failure in comorbid patients to be 2.93 (95% CI; 1.18–7.23), and the aggregate risk of failure and death to be 1.69 (95% CI; 1.36–2.12). The risk of relapse or reinfection by either the same bacterium or a different strain in these patients is also higher.22
Sullivan and Ben Amor found that developing countries have a higher prevalence of patients with both TB and DM. They go on to describe how the existence of a systemic disease added to the aetiological factors that affect treatment prognosis in TB, such as poverty, malnutrition and pollution, further complicates the outlook for these patients.34
Both authors recommend extending the DOTS tuberculosis strategy to include diabetes as a means of managing both diseases simultaneously; at least while TB therapy lasts. Once TB is resolved, they recommend channelling patients into specific diabetes management programmes.34
Tuberculosis and diabetes in MexicoMeasures to control tuberculosis in Mexico were introduced in the 1930s and implemented nationwide. Nevertheless, prevalence of the disease has increased as a result of increased immigration and HIV infection. Added to this, diabetes is currently among the leading causes of death in Mexico, and estimates suggest that the disease will affect 11.7 million inhabitants by 2025.35
According to Jeon and Murray, diabetes triples the risk of developing TB. In Mexico, around 6% of the population suffers from diabetes. More importantly, DM accounts for nearly 67% of active TB cases among people with diabetes, and 11% of cases among the entire Mexican population.30
In the same vein, the prospective study published by Jiménez-Corona in TB patients in the south of Mexico from 1995 to 2010 found that nearly 30% of patients with TB were also affected by DM. The authors also found that social and economic differences in the Mexican population are the main obstacle to correct diagnosis, as a higher socioeconomic level facilitates the diagnosis of both diseases. This circumstance can distort the epidemiological data on Mexico.22
According to Ponce de Leon et al., patients with DM are 6 times more at risk of developing TB than those without. The authors discuss the same problem reported in other studies, i.e., socioeconomic differences could hamper access to health services, and this in turn affects the number of patients diagnosed with both diseases.36
The authors claim that DM is a greater risk than HIV due to the higher prevalence of diabetes revealed by the Mexican National Health Survey ENSA2000 (DM 8.18% vs. HIV 0.1% in the over-15 age group). As DM affects the transmission and reactivation of TB infection, the authors recommend correct implementation of DOTS and early diagnosis.36
ConclusionsThe studies analysed show that diabetes drastically affects the clinical course of tuberculosis, with more severe clinical manifestations and poorer prognosis and treatment efficacy. In the absence of international guidelines on the management and control of comorbid tuberculosis and diabetes, experts must establish a strategy for treating both diseases simultaneously, and take preventive action by introducing strategic measures to prevent comorbidity in developing countries such as Mexico.
Conflict of interestThe authors declare that they have no conflict of interests.