To establish whether the palliative prognostic index (PPI), the Charlson comorbidity index (CCI) or other factors are predictors of survival for patients with ALL undergoing palliative care.
Materials and methodsRetrospective cohort study of patients diagnosed with ALL undergoing palliative care. We analysed variables at the time of diagnosis (age, WBC count, and risk type), chemotherapy regimens received, PPI, CCI and transfusion requirements at time palliative care was started.
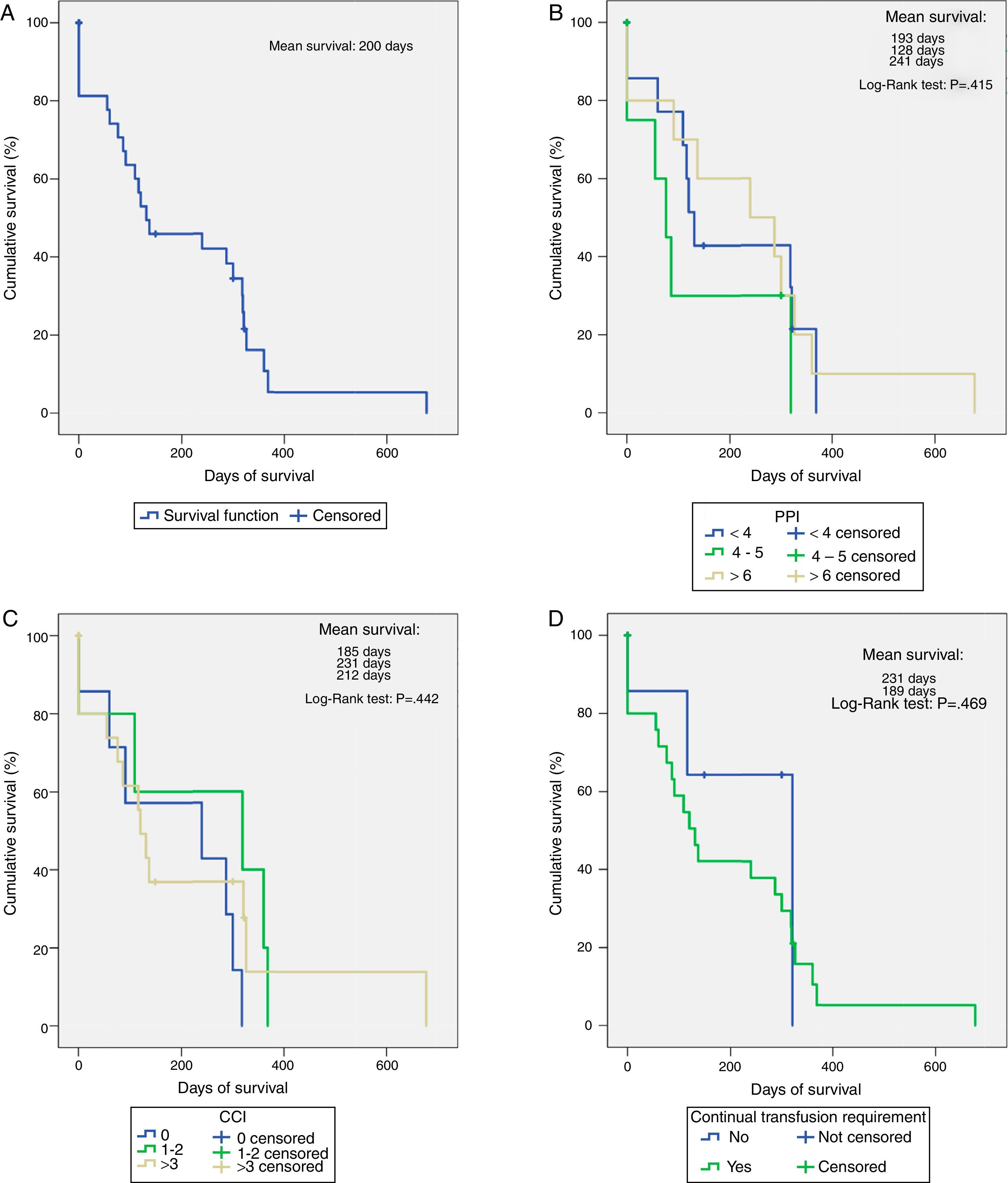
ResultsWe studied 32 patients with a mean age of 37 (18–75) years. Fourteen cases had a PPI=0 (43.8%). 62.5% (n=20) with a CCI>3 had high odds of dying within 10 years. The median survival was 200 days, unaffected by any of the factors analysed.
DiscussionNeither PPI, CCI, nor the other studied factors effectively predicted survival. Scales will have to be adapted or new predictive scales devised specifically for patients with ALL.
Establecer si el índice pronóstico paliativo (IPP), el índice de comorbilidad de Charlson (ICC) u otros factores son predictores de sobrevida en pacientes con LLA sometidos a terapia paliativa.
Material y métodosCohorte retrospectiva de pacientes con diagnóstico de LLA sometidos a terapia paliativa. Se analizaron variables al momento del diagnóstico (edad, cifra de leucocitos, tipo de riesgo), esquemas recibidos, IPP e ICC al momento de iniciar tratamiento paliativo, así como los requerimientos transfusionales.
ResultadosSe estudiaron 32 pacientes con edad promedio de 37 (18–75) años. Catorce casos obtuvieron un IPP de 0 (43.8%). El 62.5% (n=20) con ICC >3 tenía altas probabilidades de morir en menos de 10 años. La media de supervivencia fue de 200 días, sin afectarse significativamente por ninguno de los factores analizados.
DiscusiónIPP, ICC, ni otros factores predijeron efectivamente la sobrevida. Será necesario adecuar estas escalas o idear nuevas específicamente para LLA.
Acute lymphoblastic leukaemia (ALL) is a precursor B- or T-cell lymphoproliferative cancer whose treatment requires sequential doses of chemotherapy, haematopoietic stem cell transplants, monoclonal antibodies, or immunotherapy.1–3 Despite advances in treatment, in most trials 5-year survival is under 50%, but as 75% of cases have markers of poor prognosis, their survival is only 25%.4 The objective to meet for all treatment regimens is to reduce the presence of cancer cells to undetectable levels, which is called complete remission (CR), and it is obtained in 80% of patients on average around the world; however 50% of individuals in CR will relapse in the first two years after treatment, mostly associated with cytogenetic alterations.5,6 In the MRCUKALL12/ECOG 293 protocol, Fielding et al. reported that 5-year survival after a relapse is only 7%.7 Oriol et al. also reported that the mean reported survival after a relapse was 4.5 months, with the duration of the first CR being the main good prognostic factor.8
It is because of these discouraging results that the need for end-of-life-care has been increasing for most haematologic cancers, but to date only 18% of patients have access to it.9 As they behave differently from solid tumours, it is difficult to know precisely when a patient with a haematologic cancer should be considered a candidate for palliative care, either at the time of diagnosis given severe symptoms or after several treatment failures.10,11 Strictly speaking, palliative care includes measures such as pain management and home medical assistance without the use of aggressive chemotherapy, preferring low-intensity regimens with the aim of improving quality of life.12 Several indices have been used to assess the possibility of early death in terminal patients; the main ones are the Palliative Prognostic Score (PaP), the palliative prognostic index (PPI), and the Charlson Comorbidity Index (CCI). The latter two are the most commonly used worldwide (Table 1).13–15 In general, the PPI takes into account clinical situations (dyspnoea, level of activity, oral tolerance, oedema, and delirium) while the CCI takes into account only the patient's comorbidities.16,17 There is little research addressing the topic of end-of-life care in particular for individuals with haematologic cancers. The study led by the group of Hui stands out, as they identified that this type of patient shows more admissions to the emergency department, hospitalisations, use of chemotherapy or admission to intensive care units in comparison with patients who received end-of-life care for solid tumours.18
Palliative prognostic index and Charlson comorbidity index.
| Palliative prognostic index (PPI)27 | Charlson comorbidity index (CCI)28 | ||
|---|---|---|---|
| Variable | Points | Comorbidity | Points |
| Level of activity | (%) | Myocardial infarction | 1 |
| 10–20 | 4 | Congestive heart failure | 1 |
| 30–50 | 2.5 | Peripheral vascular disease | 1 |
| ≥60 | 0 | Cerebrovascular disease | 1 |
| Oral intake | Dementia | 1 | |
| Severely reduced | 2.5 | Chronic lung disease | 1 |
| Moderately reduced | 1 | Connective tissue disease | 1 |
| Normal | 0 | Ulcerative disease | 1 |
| Oedema | Mild liver disease | 1 | |
| Present | 1 | Moderate or severe liver disease | 3 |
| Absent | 0 | Diabetes | 1 |
| Dyspnoea at rest | Diabetes with organ injury | 2 | |
| Present | 1 | Hemiplegia | 2 |
| Absent | 0 | Kidney disease (moderate or severe) | 2 |
| Delirium | Solid cancers | 2 | |
| Present | 1 | Leukaemias | 2 |
| Absent | 0 | Malignant lymphomas | 2 |
| Solid tumour metastasis | 6 | ||
| AIDS | 6 | ||
| Age: After 40 years, add 0.1 points for each year of age | |||
In our country, the decision to use a low-intensity treatment such as palliative care is considered in the elderly, after the disease was refractory to two or more regimens, as well as in those cases where there was an express request made by the patient.19 The main objective of this study was to analyse the impact of the PPI and CCI scores as well as other factors on the survival of acute lymphoblastic leukaemia patients who receive end-of-life care.
MethodsStudy designObservational, retrospective cohort study of ALL patients who received end-of-life care. The analysis included clinical variables at the time of diagnosis (age>35 years, WBC count>30×103mcL−1, type of risk), type of treatment (intensive or standard), number of regimens received, and PPI and CCI scores (Table 1) at the time palliative care was started. In addition the ECOG (Eastern Cooperative Oncology Group) score19 at the time of diagnosis and the transfusion requirements was analysed.
PatientsAcute lymphoblastic leukaemia patients who were treated with several institutional chemotherapy regimens between 2013 and 2014 and who were therefore considered candidates for a less intensive regiment or palliative care were included. The decision to start palliative care or to continue with aggressive chemotherapy regimens was based on the clinical situation and the treating physician's recommendation, as long as it was in agreement with the patient's decision.
Palliative care included transfusion support should the haemoglobin figures be <8g/dL; platelet concentrates in the case of thrombocytopaenia<20×103mL−1; and antibiotics or analgesics were added if necessary. The PPI and CCI prognostic indices determinations were analysed once it was decided to use low-intensity palliative care.
Low-intensity palliative carePatients on palliative care received the low-intensity CVBP chemotherapy regimen which consists of administering: Cyclophosphamide 750mg/m2 subcutaneously (SC), vincristine 1.2mg/m2 SC, bleomycin 10IU/m2 SC, all on day +1, and prednisone 50mg/m2 SC during the first 5 days of treatment. The administration is repeated every 21 days. If necessary, granulocyte-colony stimulating factor is also administered.
Ethical considerationsThe research was conducted in accordance with the ethical principles described in the 1975 Declaration of Helsinki and subsequent updates, as well as in line with the current recommendations issued by the Mexican Ministry of Health. The study was presented to and approved by the Ethics and Research Committees of the Hospital General de Mexico (record: DI/15/204/03/54).
Statistical analysisThe statistics software package IBM SPSS version 23.0 was used (Armonk, NY: IBM Corp.). The relative risk was estimated for each variable, using the Chi-squared test to test the hypothesis. A p value <0.05 was considered significant with a 95% confidence interval. A Kaplan–Meier curve was used to analyse survival, using the log-rank test to establish the differences between classes.
ResultsA total of 32 patients considered outside intensive treatment and thus candidates for low-intensity chemotherapy were studied. Of all the cases, 56.3% were male (n=18) and 43.8% were female (n=14). The mean age was 37 years (range 18–75 years). The mean age of the female patients was significantly higher in comparison with male patients (43 vs 32, p=0.054, 95% CI).
According to the risk criteria, most of the patients had a high risk of relapse (68.8×103mcL−1). The general characteristics of the patients, as well as the blood count values at diagnosis and at the time of starting palliative care, are described in Table 2.
Clinical/biochemistry characteristics at the time of diagnosis and relapse of ALL patients treated at the Hospital General de México, 2013–2014.
| Characteristics at the time of diagnosis | Mean | Range |
|---|---|---|
| Age (years) | 37.34 | 18–75 |
| Age by sex (years) | ||
| Male (n=18) | 43 | 22–75 |
| Female (n=14) | 18–56 | |
| White blood cell count (×103mcL−1) | 18.34 | 0.5–279 |
| Haemoglobin (g/dL) | 9.43 | 5.5–16 |
| Platelets (×103mcL−1) | 127.62 | 1–482 |
| Lactate dehydrogenase (IU/L) | 317.53 | 126–604 |
| Albumin (g/dL) | 2.53 | 0–4 |
| Characteristics when starting palliative care | n | (%) |
|---|---|---|
| Transfusion support | 25 | 78.1 |
| Dyspnoea | 8 | 25 |
| Oedema | 11 | 34.4 |
| Type of risk | ||
| High | 22 | 68.8 |
| Standard | 10 | 31.3 |
| Treatment intensity | ||
| High | 3 | 9.4 |
| Low-intensity | 1 | 3.1 |
| Standard | 19 | 59.4 |
| Intensive | 9 | 28.1 |
| Regimens received | ||
| 1 | 9 | 28.1 |
| 2 | 16 | 50 |
| 3 | 7 | 21.9 |
| Time between diagnosis and relapse (days) | 167.09 | 0–686 |
Most of the patients received moderate-intensity chemotherapy in cycles (institutional protocol HGMLA07) (n=19, 59.4%), 28.1% (n=9) received higher-intensity protocols (Hyper-CVAD), 9.4% (n=3) alternative protocols including mitoxantrone, cytarabine, and etoposide and only 3.1% (n=1) received low-intensity chemotherapy from the time of diagnosis due to the performance status of the patients.
Patient characteristics at the time of starting palliative careAt the time of starting end-of-life care, 71.9% (n=23) had a bone marrow relapse and only 28.1% (n=9) were refractory to the first treatment regimen. Of all of treatment regimens, most had two treatment regimens (n=16, 50%), 28.1% (n=9) only had one treatment regimen and decided on a lower intensity regiment, 21.9% (n=7) had 3 or more previous therapies at the time of considering palliative or low-intensive care (Table 2).
Most of the patients had an ECOG score of 0 (n=15, 46.9%), followed by a score of 2 (n=11, 34.4%), only 6.3% (n=2) had an ECOG score of 3. As for transfusion support, 78.1% (n=25) required continuous transfusion support of both platelet concentrates and red blood cell concentrates (Table 2).
PPI and CCIA large portion of the patients had a CCI>3 (n=20, 62.5%) i.e. more than half of the study population has a high chance (>50%) of dying in the next 10 years, and the rest has a moderate chance (CCI: 1–2, 11–50%) or lower (CCI: 0, <10%) of dying in the same period of time.
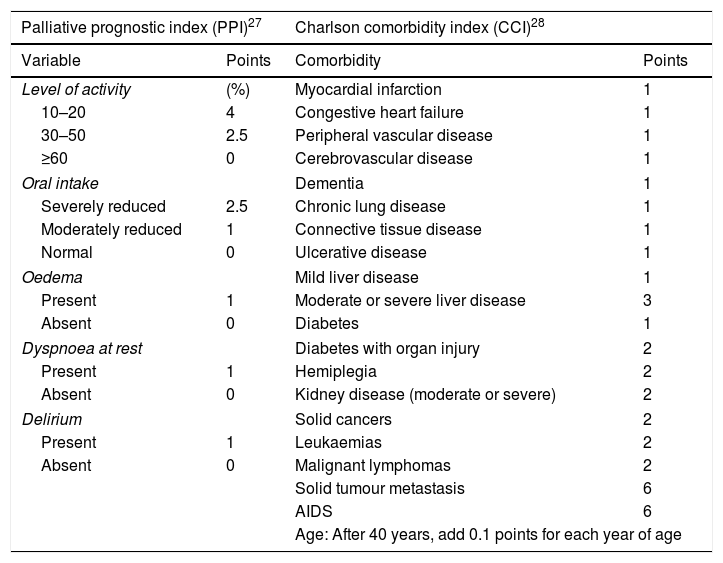
As for the PPI, a significant proportion had a score under 4 (n=14, 43.8%), therefore their chance of survival was higher in the PPI time period (>6 weeks). One third (n=10, 31.2%) had a score above 6, with a calculated survival of less than 3 weeks, and lastly 8 patients (25%) had an estimated survival of 6 weeks (4<PPI>6) (Table 3).
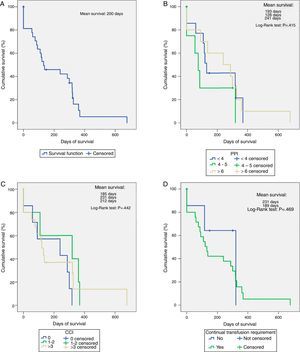
SurvivalMean overall survival was 200 days (Fig. 1A). The PPI (Fig. 1B) and CCI scores (Fig. 1C) did not significantly influence survival (p>0.05) during the stratified analysis.
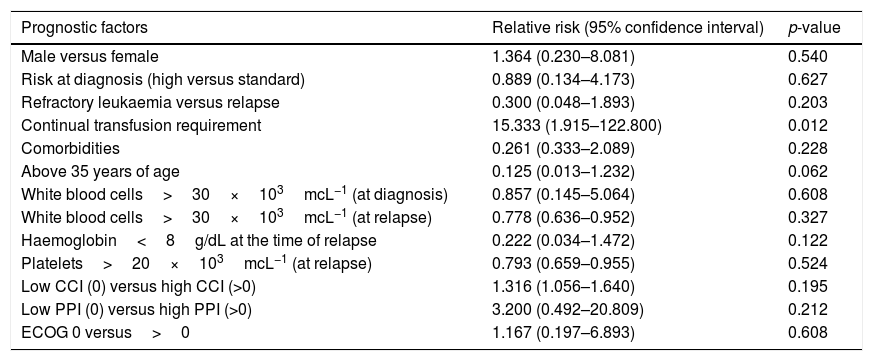
Factors associated with survivalOf all the variables analysed as possible factors that decrease survival, only the requirement for continuous transfusions showed a strong impact (RR: 15.33, 95% CI: 1.915–122.800, p=0.012) (Table 4). After creating the survival curves based on this factor, 7 patients who did not receive continuous transfusions had a mean survival of 231 days, higher than the rest of the cases (n=25, 189 days), although this difference was not statistically significant (p=0.469) (Fig. 1D).
Factors associated with survival in ALL patients in end-of-life care at the Hospital General de México, 2013–2014.
| Prognostic factors | Relative risk (95% confidence interval) | p-value |
|---|---|---|
| Male versus female | 1.364 (0.230–8.081) | 0.540 |
| Risk at diagnosis (high versus standard) | 0.889 (0.134–4.173) | 0.627 |
| Refractory leukaemia versus relapse | 0.300 (0.048–1.893) | 0.203 |
| Continual transfusion requirement | 15.333 (1.915–122.800) | 0.012 |
| Comorbidities | 0.261 (0.333–2.089) | 0.228 |
| Above 35 years of age | 0.125 (0.013–1.232) | 0.062 |
| White blood cells>30×103mcL−1 (at diagnosis) | 0.857 (0.145–5.064) | 0.608 |
| White blood cells>30×103mcL−1 (at relapse) | 0.778 (0.636–0.952) | 0.327 |
| Haemoglobin<8g/dL at the time of relapse | 0.222 (0.034–1.472) | 0.122 |
| Platelets>20×103mcL−1 (at relapse) | 0.793 (0.659–0.955) | 0.524 |
| Low CCI (0) versus high CCI (>0) | 1.316 (1.056–1.640) | 0.195 |
| Low PPI (0) versus high PPI (>0) | 3.200 (0.492–20.809) | 0.212 |
| ECOG 0 versus>0 | 1.167 (0.197–6.893) | 0.608 |
The main limiting factor for achieving good results with paediatric chemotherapy regimens are relapses, conditioned by an elevated tumour load at diagnosis or by cytogenetic alterations of poor prognosis.20 The scenario after relapse is completely different, as only 50% of the patients will reach a second complete remission and the remaining will be refractory or will die during treatment.20,21 It is in these refractory patients in which doubt arises regarding the real benefit of submitting these patients to highly aggressive regimens, compromising their quality of life, or instead providing palliative care, giving better quality of life to not only the patient, but also to their family members by preventing exhaustion in stressful situations causes by the prolonged hospital stay.22 Unlike what happens when assessing patients with solid tumours, there are very few scales that provide a recommendation for making the decision to submit patients with haematologic cancers to a new high-intensity regimen or to opt for end-of-life care.22,23 In all likelihood, the PPI is the most described and validated worldwide, although in patients with solid tumours. Hung et al. found that it was extremely useful prognostically to make two PPI measurements in terminal patients spaced one week apart, especially when the PPI is >6.24 Cheng et al. identified that patients with a score>6 showed a means survival of 7 days, with a positive predictive value of 81% for predicting death in a 21-day interval.16 Similarly, Stone et al., reported a positive predictive value of 91% for predicting death in a 6-week interval.25 Recently, Baba et al. evaluated different prognostic indices (PaP score, Delirium-Palliative Prognostic Score, PPI, modified Prognosis in Palliative Care Study predictor model) in 4 groups of patients including those still undergoing chemotherapy. They concluded that the PPI is useful for daily practice in patients with no intensive treatments or procedures, unlike the PaP score or the Delirium-PaP score which can be used in those who require undergoing a higher number of procedures.26 The work of Trujillo-Cariño must not be forgotten, as she validated the PPI in the Mexican population, obtaining results similar to those reported.27 Nevertheless, when we applied the PPI to our series, we did not find significant differences in terms of survival, not even in those classified as high risk. This is possibly due to the fact that unlike solid tumours, it is rare for a patient with refractory leukaemia to have limitations on oral intake or delirium, even in those cases with central nervous system infiltration it is very rare for this to occur; therefore despite having a high-risk classification and high odds of dying, their PPI will remain low.
As for CCI, it has been applied to a wide range of end-stage diseases, not only cancers.28,29 One of the main advantages over PPI is it takes the patient's age into account to adjust the survival prediction, a key point in ALL patients in whom age has been demonstrated to be a significant impact factor on survival in all ALL patients, but particular in those who relapse.30,31 A study applying the CCI to patients with chronic myeloid leukaemia (CML) was recently conducted using this concept. The researchers of the German CML Study Group concluded that the CCI was highly useful for predicting mortality; even when it was not age-adjusted the comorbidities alone reduced survival.32 It thus agrees with a prior study from the same groups but with chronic lymphoid leukaemia patients to whom the CCI was also applied. The presence of two or more comorbidities negatively impacted the risk of death.33
Although in the end PPI and CCI are used to predict mortality, there is a clear difference between the two. PPI is based on the patient's signs and symptoms at the time it is applied, thus enabling it to indirectly identify how much quality of life is affected, with the limitation of not discriminating if the symptoms are due to the underlying pathology, complications, treatment side effects, or present comorbidities.26,27 For the CCI, on the other hand, a more in-depth review of the patient's history is required in order to take into account the diagnosis of concomitant diseases, many of which require laboratory testing, giving the scale greater objectivity at the cost of not providing precise information on the patient's limitations and how much their quality of life of life has decreased.27,29
Lastly, it can be concluded that the PPI and CCI scores are not correlated with the survival of ALL patients in palliative care; however survival was also not affected by other factors typically used as predictors and widely described in the medical literature, such as the white blood cell or haemoglobin count. Therefore additional research will be required to determine the factors that truly have an impact on survival and these scales can be modified or new scales designed for this type of patient with a different clinical course than that of solid tumours where they have been successfully used.
Ethical disclosureProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThis work had no external source of funding and was conducted with existing resources at the Hospital General de México “Dr. Eduardo Liceaga”.
Conflict of interestsThe authors declare that they have no conflicts of interest.